Product Overview
Arginine HCl Injection is a compounded sterile solution of the amino acid L-arginine hydrochloride, intended for intravenous use. It is primarily utilized as an intravenous stimulant to the pituitary gland for the release of human growth hormone (hGH), aiding in the evaluation of pituitary growth hormone reserve in patients with suspected deficiency. This formulation was formerly known by the brand name R-Gene® 10 when used for growth hormone stimulation testing. It may be employed as a diagnostic aid in various conditions related to pituitary function and growth, such as panhypopituitarism, pituitary dwarfism, acromegaly, and other disorders of growth and stature. In addition to its diagnostic role, intravenous arginine has been used in certain metabolic situations, for example, to help manage acute hyperammonemia in patients with urea cycle disorders, although such applications are considered off-label and are pursued under specialized medical supervision.[1]
As a compounded medication, Arginine HCl Injection is available only by prescription and is prepared in regulated compounding facilities. Depending on clinical needs, it may be obtained from a 503A compounding pharmacy (for patient-specific preparation) or from a 503B outsourcing facility that adheres to FDA oversights for bulk compounding. Because it is not a commercially manufactured drug, this formulation has not undergone the FDA approval process; its use is confined to qualified healthcare professionals and individualized therapeutic plans.[2]
L-arginine itself is a naturally occurring amino acid present in many protein-rich foods, and he body can normally synthesize sufficient amounts for general needs. However, the doses provided by Arginine HCl Injection far exceed dietary levels and have pharmacological effects. This high-dose intravenous administration influences multiple hysiological pathways and must be used under strict medical supervision to ensure safety and appropriate monitoring. In clinical practice, arginine infusions are administered in a controlled setting, with patients’ vital signs and relevant laboratory values monitored due to the formulation’s potent systemic effects.[3]
In growth hormone stimulation testing, a standard adult dosage of arginine HCl is 30 g administered by intravenous infusion over 30 minutes. Pediatric dosing is weight-based (approximately 0.5 g of arginine per kg body weight, infused over 30 minutes), not to exceed a total of 30 g.[17] This protocol typically uses a 10% arginine HCl solution (for example, 300 mL of a 10% solution contains 30 g of arginine). During the test, serial blood samples are drawn at baseline and at regular intervals after the infusion to measure the growth hormone response to arginine.
For other indications, dosing varies. In acute hyperammonemia (an off label use in urea cycle disorders), arginine may be given in much higher doses (often an initial bolus followed by a continuous infusion) tailored to the patient’s weight and ammonia levels, under intensive care supervision. All dosing regimens are individualized by the treating physician, and arginine therapy is initiated and adjusted with careful monitoring to ensure safety and effectiveness.
L-arginine is a biochemical precursor for nitric oxide (NO), a potent vasodilator. Large doses of arginine delivered intravenously can significantly increase NO production, which relaxes vascular smooth muscle and widens blood vessels. By increasing NO availability, arginine infusion may improve endothelial function and blood flow, potentially reducing vascular resistance and lowering blood pressure under certain conditions. This vasodilatory mechanism contributes to arginine’s influence on cardiovascular dynamics. NO also has numerous signaling roles in the body, influencing platelet function and immune responses, which may contribute to some of arginine’s observed effects.[4]
Arginine also exerts notable endocrine effects. An arginine infusion may stimulate the anterior pituitary to secrete growth hormone, likely by inhibiting the release of somatostatin (the endogenous growth hormone inhibitor). By suppressing hypothalamic somatostatin tone, arginine removes an inhibitory brake on growth hormone secretion.[5] Consequently, individuals with intact pituitary function respond to arginine with a pronounced rise in plasma growth hormone levels, whereas patients with pituitary impairment exhibit a blunted or absent response. Arginine may also transiently stimulate the release of other hormones (for example, insulin), reflecting its broad impact on metabolic and endocrine pathways. This mechanism underlies the use of arginine in growth hormone stimulation tests as an indicator of pituitary reserve for hGH.[5]
Additionally, arginine plays a key role in the urea cycle, the metabolic pathway in the liver that converts ammonia into urea for excretion. In patients with urea cycle disorders, endogenous arginine synthesis is deficient; administering arginine HCl provides a critical substrate to facilitate the conversion of toxic ammonia to urea, thereby aiding ammonia detoxification. This mechanism explains the therapeutic rationale for using arginine infusions (often alongside other agents) in cases of acute hyperammonemia; by supplying the missing urea cycle intermediate, arginine may help reduce dangerously elevated ammonia levels in these conditions.[6]
In summary, arginine’s pharmacological effects span multiple systems, vascular, endocrine, and metabolic, underscoring the need for careful administration tailored to the therapeutic goal and close clinical monitoring of its effects.[5]
Arginine HCl Injection is contraindicated in individuals with a known hypersensitivity or allergy to arginine or any of the formulation’s components. Patients who have experienced any form of arginine-induced hypersensitivity reaction (such as anaphylaxis, significant rash, or other allergic manifestations) should not receive this preparation.[7]
Use of L-arginine is generally avoided in patients who have recently suffered a myocardial infarction (heart attack). Clinical evidence suggests that post-MI arginine therapy may worsen outcomes; in a notable trial, patients given arginine following an acute MI had a higher mortality rate compared to those given placebo, leading experts to advise against arginine supplementation in the immediate post-infarction period.[8]
Additional precautions apply to certain preexisting conditions. For example, individuals with the rare genetic disorder guanidinoacetate methyltransferase (GAMT) deficiency are typically advised to avoid arginine, because excess arginine can exacerbate metabolic imbalances in this condition. Pediatric and adolescent patients generally should not receive high-dose arginine except under specialist supervision, and similarly, older adults are approached with caution given limited clinical data and potentially heightened sensitivity in that population.[9]
Arginine should also be used carefully in patients with significant renal impairment or vere hepatic disease. The amino acid load from arginine HCl can disturb electrolyte balances, for instance, infusions have been observed to provoke hyperkalemia (elevated potassium levels) in patients with compromised liver and kidney function, and serious adverse events (including fatal cardiac arrhythmias) have been reported in such settings. Therefore, in patients with renal or hepatic insufficiency (or both), arginine is generally avoided or only administered with extreme caution under close monitoring.[10]
Arginine may also exacerbate certain conditions such as asthma or active allergies, given its influence on inflammatory and immune pathways. Individuals with a history of asthma or atopic allergies should use arginine only under medical guidance, and those prone to herpes (cold sores) are cautioned that high arginine levels can potentially trigger viral reactivations.[11]
L-arginine has the potential to interact with a number of medications and supplements. Because arginine can lower blood pressure, it may have additive hypotensive effects if combined with antihypertensive drugs (e.g., ACE inhibitors, angiotensin receptor blockers, beta-blockers, or diuretics) or with other vasodilators like nitrates and phosphodiesterase 5 inhibitors (such as nitroglycerin or sildenafil). Concurrent use of arginine alongside these agents could result in an excessive drop in blood pressure, leading to lightheadedness or even fainting. Arginine might also affect blood sugar control, it can enhance insulin release and sensitivity, so in patients on antidiabetic medications, combining arginine could increase the risk of hypoglycemia and necessitates close glucose monitoring. Notably, most interactions of arginine are pharmacodynamic rather than pharmacokinetic; arginine does not directly alter drug metabolism, but it can compound the physiological effects of other agents on blood pressure, blood glucose, or coagulation.[12]
In addition, arginine exhibits mild anticoagulant and platelet-inhibiting properties, meaning that use with blood-thinning drugs or supplements (like warfarin, aspirin, or high-dose fish oil) might heighten bleeding tendencies. Combining arginine with other substances that lower blood pressure (for example, garlic or coenzyme Q10) could similarly amplify hypotensive effects. Furthermore, co-administration of arginine with potassium-sparing diuretics (e.g., spironolactone, amiloride) is cautioned, as both can increase serum potassium levels and together may precipitate hyperkalemia (high potassium), which in turn may lead to dangerous heart arrhythmias. Even certain emergency medications (for example, the vasopressor isoproterenol) could have amplified effects if arginine is present; therefore, arginine should be used with caution or discontinued around the time of such treatments. It is generally recommended to consult a healthcare provider before combining arginine with any other medications or supplements to mitigate potential interaction risks.[13]
Most patients tolerate arginine infusions, but side effects can occur, especially at higher doses. Commonly reported adverse effects include nausea, vomiting, abdominal cramping, bloating, and diarrhea, and occasionally numbness or tingling sensations. Patients may also experience flushing or headache due to arginine’s vasodilating effects. Some people report a sense of dizziness or lightheadedness during the infusion, which is consistent with transient blood pressure lowering. Irritation at the infusion site or vein (such as redness or pain along the vein) is another relatively frequent side effect, given the hypertonic nature of the arginine HCl solution.[14]
More serious side effects are less common but medically important. Arginine infusions can provoke allergic reactions in susceptible individuals, for example, hives, wheezing, or even anaphylaxis (severe allergic shock) have been reported. A large or rapid infusion could potentially lead to a marked drop in blood pressure, potentially leading to fainting or cardiovascular collapse in rare cases. There are also case reports of arginine contributing to electrolyte disturbances; as noted, arginine can raise potassium levels, and in patients with underlying liver or kidney issues this has led to dangerous hyperkalemia and heart rhythm disturbances. Local complications have occurred when arginine extravasates (leaks outside the vein), including tissue damage or necrosis at the injection site requiring surgical intervention. Overall, such severe reactions are rare; arginine’s safety profile is quite favorable overall, especially when standard dosing guidelines are followed carefully.[15]
Overall, arginine infusions are considered safe for most individuals when administered by trained professionals. Most side effects are transient and mild, and serious complications are infrequent. Proper infusion technique (dilution and controlled infusion rate) and patient monitoring are important to minimize the risk of adverse reactions.[15]
Arginine HCl Injection is not routinely used during pregnancy unless clearly indicated. Animal reproductive studies have not demonstrated harm at high doses, but adequate controlled studies in pregnant women are lacking. Some research suggests potential benefits of arginine supplementation in pregnancy-related complications such as pre-eclampsia (high blood pressure in pregnancy), for example, arginine infusions have been associated with lowered blood pressure and improved outcomes in women with pre-eclampsia. However, due to insufficient safety data, arginine should be used during pregnancy only if recommended by and under the supervision of a qualified healthcare provider.[16]
It is also not well established how much arginine, if any, passes into breast milk; while amino acids in general are present in breast milk, caution is advised if arginine therapy is considered in breastfeeding mothers.[16]
Arginine HCl Injection should be stored at a controlled room temperature. The manufacturer recommends storage at approximately 25 °C (77 °F). As a compounded sterile preparation, the injection should be kept in its sealed vial under sterile conditions until use, and it should be used before its assigned beyond-use date. The product should be protected from excessive heat and direct light during storage, and it should only be handled by authorized personnel under proper storage guidelines.[18]
- Drugs..com. (2025, May 27). Arginine (L-Arginine) - Professional Patient Information. Retrieved from https://www.drugs.com/ppa/arginine.html
- U.S. Food and Drug Administration. (2018). FD&C Act Provisions that Apply to Human Drug Compounding: Sections 503A and 503B. Retrieved from
- https://www.fda.gov/drugs/human-drug-compounding/fdc-act-provisions-apply-human drug-compounding
- National Library of Medicine. (2021). L-Arginine - Dietary Supplement Fact Sheet. Retrieved from https://medlineplus.gov/druginfo/natural/875.html
- Koga, Y., Povalko, N., Inoue, E., et al. (2018). Therapeutic regimen of L-arginine for MELAS: 9-year, prospective, multicenter, clinical research. Journal of Neurology, 265(12), 2861-2874. https://doi.org/10.1007/s00415-018-9057-7
- Goli, P., Yazdi, M., Heidari-Beni, M., & Kelishadi, R. (2022). Growth hormone response to L-arginine alone and combined with GHRH: A systematic review and meta-analysis. International Journal of Endocrinology, 2022, 8739289.
- https://doi.org/10.1155/2022/8739289
- U.S. Food and Drug Administration. (2022, November 23). List of bulk drug substances for which there is a clinical need under Section 503B of the FD&C Act (Proposed Rule). Federal Register, 87(225), 71645-71665.
- RxList. (2025). Arginine (L-arginine) - Uses, Side Effects, and Warnings. Retrieved from https://www.rxlist.com/arginine/generic-drug.htm
- Schulman, S. P., et al. (2006). L-arginine therapy in acute myocardial infarction: The VINTAGE MI trial. JAMA, 295(1), 58-64. https://doi.org/10.1001/jama.295.1.58 9.Cleveland Clinic. (2022, March 22). L-Arginine: Benefits, Uses and Side Effects. Retrieved from https://my.clevelandclinic.org/health/drugs/22536-l-arginine
- Bushinsky, D. A., & Gennari, F. J. (1978). Life-threatening hyperkalemia induced by arginine. Annals of Internal Medicine, 89(5), 632-634. https://doi.org/10.7326/0003-4819- 89-5-632
- Mayo Clinic Staff. (n.d.). L-arginine - Overview and Safety. Mayo Clinic. Retrieved June 2025, from https://www.mayoclinic.org/healthy-lifestyle/consumer-health/expert answers/l-arginine
- Kubala, J. (2025, March 11). Everything You Need to Know About L-Arginine. Healthline. Retrieved from https://www.healthline.com/nutrition/l-arginine
- National Library of Medicine. (2021). L-Arginine - Interactions and Drug Effects. Retrieved from https://medlineplus.gov/druginfo/natural/875.html#safety 14.Cunha, J. P. (2022). R-Gene 10 (Arginine Hydrochloride) Injection - Drug Summary. RxList. Retrieved from https://www.rxlist.com/r-gene-10-drug.htm
- Pfizer, Inc. (2010). R-Gene 10 (Arginine Hydrochloride Injection, USP) - Prescribing Information. U.S. Food and Drug Administration. Retrieved from
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/016931s031lbl.pdf 16.Drugs..com. (2024, October 22). L-Arginine - Uses, Benefits & Dosage (Natural Medicines). Retrieved from https://www.drugs.com/npp/l-arginine.html
- Pharmac (New Zealand). (2009). Arginine Hydrochloride Injection 5% - Data Sheet. Retrieved from http://e-lactancia.org/media/papers/DS-Arginine-2009.pdf 18.Pfizer, Inc. (2010). R-Gene 10 (Arginine Hydrochloride Injection) - Storage Guidelines. (Labeling Archive). Retrieved from
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/016931s031lbl.pdf 19.Mount Sinai Hospital. (n.d.). Growth Hormone Stimulation Test. Retrieved June 2025, from https://www.mountsinai.org/health-library/tests/growth-hormone-stimulation-test 20.National Library of Medicine. (n.d.). Growth Hormone Tests - Lab Test Information. Retrieved June 2025, from https://medlineplus.gov/lab-tests/growth-hormone-tests 21.Mayo Clinic. (n.d.). L-Arginine - Supplement Safety. Retrieved June 2025, from https://www.mayoclinic.org/healthy-lifestyle/consumer-health/expert-answers/l-arginine 22.National Library of Medicine. (n.d.). L-Arginine - Side Effects (MedlinePlus). Retrieved June 2025, from https://medlineplus.gov/druginfo/natural/875.html#safety 23.Pfizer, Inc. (2010). R-Gene 10 - Adverse Reactions. (Prescribing Information). Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/016931s031lbl.pdf 24.Cleveland Clinic. (2022). L-Arginine - Precautions. Cleveland Clinic Health Library. Retrieved from https://my.clevelandclinic.org/health/drugs/22536-l-arginine 25.Drugs..com. (2024). L-Arginine in Pregnancy and Lactation. Retrieved June 2025, from https://www.drugs.com/npp/l-arginine.html
- Kubala, J. (2025, March 11). L-Arginine - Drug Interactions. Healthline. Retrieved from https://www.healthline.com/nutrition/l-arginine
- Mount Sinai Hospital. (n.d.). Growth Hormone Stimulation Test - Procedure and Preparation. Retrieved June 2025, from https://www.mountsinai.org/health library/tests/growth-hormone-stimulation-test
- Drugs..com. (2025, May 27). L-Arginine Supplements vs. Intravenous Arginine (Q&A). Retrieved from https://www.drugs.com/ppa/arginine.html
What is Arginine HCl Injection and what is it used for?
Arginine HCl Injection is a sterile intravenous formulation of the amino acid L-arginine. It is primarily used as a diagnostic tool to stimulate growth hormone release in a growth hormone stimulation test. In this test, arginine is infused to evaluate pituitary function and determine if the body can produce adequate growth hormone. The injection may also be used in certain specialized treatments (for example, in metabolic or critical care settings), but its main indication is diagnostic.[19]
How does Arginine HCl Injection work?
Arginine HCl Injection works by providing a large amount of arginine into the bloodstream, which triggers physiological responses. Arginine stimulates the pituitary gland to secrete growth hormone by suppressing an inhibitory hormone (somatostatin). It also serves as a precursor to nitric oxide, causing blood vessels to dilate. Through these mechanisms, arginine infusion can raise growth hormone levels (for diagnostic testing) and improve blood flow.[20]
Is Arginine HCl Injection safe?
When administered by medical professionals in appropriate doses, Arginine HCl Injection is considered safe for most people. It has been used for many years in diagnostic testing. Most side effects are mild and transient. However, like any medication, it must be used with caution in certain individuals. Overall, its safety profile is favorable, especially under proper medical supervision.[21]
What are the common side effects?
Common side effects of arginine infusions tend to be minor. They can include flushing (a feeling of warmth and redness, usually in the face), headache, nausea, vomiting, or abdominal discomfort. Some people experience a drop in blood pressure during the infusion, which might cause lightheadedness. Irritation at the IV site (such as redness or vein soreness) can also occur due to the solution’s high concentration.[22] These effects are generally temporary and resolve after the infusion.
Can it cause any serious side effects?
Serious adverse reactions to arginine are uncommon. In rare cases, allergic reactions (such as hives, swelling, or even anaphylaxis) have been reported. A large or rapid infusion could potentially lead to a significant blood pressure drop. In patients with certain conditions (like severe liver or kidney impairment), arginine infusion could contribute to high potassium levels, which may affect heart rhythm. Also, if the IV leaks arginine solution into surrounding tissue, it can cause tissue irritation. Such severe outcomes are infrequent; arginine injections are usually well-tolerated when given correctly.[23]
Who should not use Arginine HCl Injection?
Individuals with a known hypersensitivity to arginine should not receive this injection. It is also typically avoided in patients who have recently had a heart attack, because arginine supplementation was associated with worse outcomes in that setting. Caution is advised in those with severe kidney or liver disease, since they may be at higher risk for electrolyte disturbances from arginine. Pediatric and elderly patients can receive arginine if needed, but it will be carefully considered and monitored by the healthcare provider.[24]
Can Arginine HCl Injection be used during pregnancy or breastfeeding?
Arginine HCl Injection is used in pregnancy only when clearly needed and prescribed by a doctor. There is limited research in pregnant women, so arginine infusions are not routine during pregnancy. In some cases (such as a pregnancy complication like pre-eclampsia), an arginine infusion might be considered under specialist supervision to help lower blood pressure, but the safety for the fetus is not fully established. As for breastfeeding, it’s not well known how much arginine from the injection could pass into breast milk. Therefore, nursing mothers would only be given arginine under close medical guidance if the benefits are deemed to outweigh any potential risks.[25]
Does Arginine HCl Injection interact with other drugs or supplements?
Yes. Arginine can interact with certain medications. For example, because it lowers blood pressure, combining it with blood pressure medications or nitrates (for chest pain) could cause an excessive drop in blood pressure. Taking arginine alongside drugs for diabetes ight increase the risk of low blood sugar. It may also enhance the effects of blood thinners or antiplatelet supplements (like warfarin or high-dose fish oil), potentially increasing bleeding risk.[26] Always inform your healthcare provider about all the other medicines or supplements you are taking, so they can manage any potential interactions.
How is the injection given, and do I need to prepare for the test?
Arginine HCl Injection is given intravenously (through an IV line). In the growth hormone stimulation test, it is typically infused over about 30 minutes. A nurse or doctor will insert an IV catheter, and multiple blood samples are drawn before, during, and after the arginine infusion to measure hormone levels. Preparation for the test usually includes fasting (not eating or drinking) for 8-12 hours beforehand so that the results are
accurate.[27] Your healthcare provider will give you specific instructions, such as whether to hold any medications prior to the test.
Is an arginine infusion the same as taking arginine pills or supplements?
No. The arginine injection delivers a much higher dose directly into the bloodstream compared to oral supplements. Oral L-arginine supplements are available over the counter and can support nitric oxide production to some degree, but when taken by mouth, much of the arginine is metabolized before reaching the bloodstream. The injection is used for specific clinical purposes (like diagnostic testing or acute treatments) that require prompt and significant increases in arginine levels. Oral supplements cannot substitute for the intravenous form in those settings.[28]
Disclaimer: This compounded medication is prepared under section 503A and 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
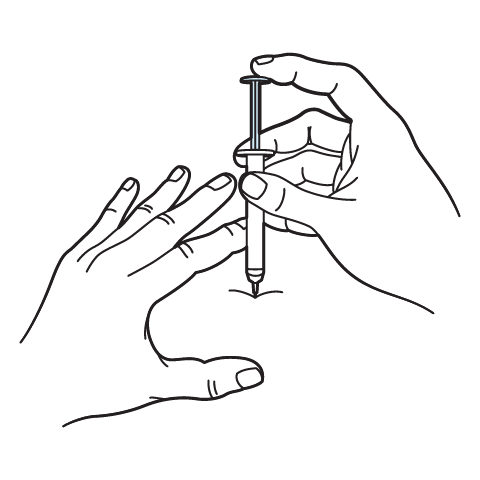
Intramuscular Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Alpha-Lipoic Acid Injection
Alpha-Lipoic Acid Injection Glycine Injection
Glycine Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection Pyridoxine HCl Injection
Pyridoxine HCl Injection BCAA Injection
BCAA Injection Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection