Product Overview
† commercial product
Bacteriostatic Water for Injection is a sterile, nonpyrogenic water solution containing 0.9% benzyl alcohol as a preservative.[1]
It is used as a diluent to dissolve or dilute medications for intravenous (IV), intramuscular (IM), or subcutaneous (SQ) injection according to the instructions of the specific drug being prepared.[1]
Benzyl alcohol acts as a bacteriostatic agent, meaning it inhibits the growth of bacteria in the solution. This allows the product to be supplied in multi-dose vials and used for repeated withdrawals, in contrast to preservative-free sterile water which is intended for single use only.[2]
By preventing bacterial proliferation, bacteriostatic water remains usable for multiple injections from the same vial when handled with proper aseptic
technique.[2]
Bacteriostatic Water is not a drug with an independent dosage; it is used only as a solvent or diluent for other injectable medications. The volume of bacteriostatic water required for reconstituting or diluting a medication depends entirely on the instructions provided for that specific medication.[10]
Each drug will have recommended dilution guidelines, for example, a powdered medication vial may specify adding a certain number of milliliters of diluent. The healthcare provider should use only the amount of bacteriostatic water needed to achieve the desired concentration for injection, following the drug manufacturer’s directions.[10]
Using the correct volume ensures the medication is properly dissolved or diluted and delivered at the intended dose. It’s important to mix thoroughly after adding the water to ensure the drug goes completely into solution. After reconstitution, the medication should be administered using the route and rate specified (IV, IM, or SC) per the drug’s guidelines.
Administration Precautions: Bacteriostatic Water for Injection should never be injected intravenously (or intra-spinally) by itself without first being mixed with a medication or appropriate solute. Because it is essentially water without sufficient solute, direct intravenous administration can cause red blood cell lysis (hemolysis) due to its hypotonic nature.[11]
To avoid this, always ensure that the final admixture is approximately isotonic, typically achieved by the drug or other diluents added to the bacteriostatic water, before injecting into the bloodstream. In practical terms, this means bacteriostatic water is only to be used to dissolve or dilute other substances, and the resulting solution should have an appropriate concentration of electrolytes or osmotic agents.
If the manufacturer of a medication specifies a particular diluent (e.g., sterile saline), bacteriostatic water should not be substituted unless approved, and if used, the osmolarity of the mixture must be considered.[11]
By adhering to these precautions, one can safely use bacteriostatic water to prepare injections without causing harm from the diluent itself.
Bacteriostatic Water has no direct pharmacological action on the body; its purpose is to safely deliver other medications.
The inclusion of benzyl alcohol (at 0.9%) imparts an antimicrobial effect by inhibiting bacterial growth and reproduction in the water. This bacteriostatic (growth-inhibiting) property, rather than a bactericidal (bacteria-killing) effect, ensures that any contaminating microbes do not multiply.
As a result, a single vial of bacteriostatic water can be used for multiple dose preparations over time (up to 28 days after opening) without significant risk of
contamination.[3]
The water itself simply serves as a solvent or vehicle for the drug and does not alter the drug’s mechanism of action.
Neonates: Bacteriostatic Water for Injection is contraindicated in neonates (newborn infants), especially premature infants. The benzyl alcohol preservative has been linked to severe toxicity in this population, including a potentially fatal condition known as “gasping syndrome.” Newborns have immature metabolic pathways and cannot efficiently metabolize benzyl alcohol, leading to its accumulation and metabolic acidosis with respiratory failure in severe cases.
Therefore, solutions containing benzyl alcohol must not be used for preparing medications for neonates; if a diluent is needed for an infant, preservative-free sterile water for injection should be used instead.
Additionally, use of bacteriostatic water is contraindicated in any patient with a known hypersensitivity to benzyl alcohol, as it could provoke an allergic reaction in such individuals.[4]
Patients with benzyl alcohol allergy should not be exposed to this diluent.
Route-specific and Other Contraindications: Bacteriostatic Water must not be used for large-volume intravenous fluid replacement or hydration therapy. The formulation is intended only for diluting medications in small quantities; using it as an IV infusion or flush solution could result in excessive benzyl alcohol intake.
It is also contraindicated for epidural or spinal (intrathecal) injection procedures. Preparations containing preservatives should not be introduced into the cerebrospinal fluid due to the risk of neurotoxic effects on neural tissue. If a medication is to be administered via the intrathecal/epidural route, only preservative-free diluents (such as sterile saline or sterile water) are appropriate.
In summary, Bacteriostatic Water for Injection should only be used as directed to dilute or dissolve drugs for injection, and it must be made approximately isotonic with the added medication or solution prior to intravenous administration.[5]
Injecting bacteriostatic water alone, without dilution, or in large volumes, is dangerous and therefore contraindicated.
Bacteriostatic Water for Injection does not have traditional drug-drug interactions since it contains no active pharmaceutical ingredient; however, there are important compatibility considerations when mixing it with other drugs.
Some medications may be incompatible with bacteriostatic water or with the benzyl alcohol preservative. For example, certain biological products or drugs (like some vaccines or delicate protein-based drugs) should not be reconstituted with bacteriostatic water because the preservative could interfere with the drug’s stability or efficacy.
Always consult the medication manufacturer instructions for dilution requirements and compatibility. If there is any uncertainty, a pharmacist should be consulted to determine the appropriate diluent.[6]
In practice, many drug vials will specify if they must be reconstituted with sterile water or saline instead of bacteriostatic water. Following these guidelines is crucial, as using an incompatible diluent could cause precipitation of the drug or inactivation of the drug’s therapeutic effect.
When used properly as a diluent, bacteriostatic water itself has minimal side effects in most patients. It is an inert vehicle, so any adverse effects are usually related to the act of injection or the medication being administered rather than the diluent.
Common local reactions may include mild pain, redness, or swelling at the injection site. Improper technique or contamination can lead to more serious localized issues such as injection site infection, abscess formation, tissue necrosis, or thrombophlebitis (vein inflammation). These injection-site reactions are generally due to mechanical irritation or microbial contamination.
Systemic side effects from the tiny amount of benzyl alcohol in each dose are exceedingly rare in adults. Notably, newborn infants are at risk of severe toxicity (as discussed in Contraindications), but in adults and older children, the small benzyl alcohol dose is very well tolerated.
If fever, chills, or other unexpected systemic reactions occur during an injection, it is likely due to the drug being delivered or possible contamination, rather than the bacteriostatic water itself. Any such adverse reaction should be evaluated by a healthcare provider.[7]
There are no adequate studies of bacteriostatic water in pregnant women, and its effects on reproduction are not well-studied.
Bacteriostatic Water for Injection is pharmacologically inactive aside from the preservative, but the safety of benzyl alcohol in pregnancy has not been established. Animal reproduction studies on bacteriostatic water (with benzyl alcohol) have not been conducted, so its potential to cause fetal harm is unknown.
For this reason, bacteriostatic water-diluted medications should be used during pregnancy only if clearly needed and if the potential benefit justifies any potential risk to the fetus.[8]
Healthcare professionals often exercise caution and may opt for preservative-free diluents in pregnant patients when possible, especially if large volumes of diluent are required.
Benzyl alcohol can cross the placenta and is also likely excreted in breast milk in small amounts. While the exposure from bacteriostatic water is very low, there is a theoretical risk that an infant could ingest benzyl alcohol through breast milk.
In practice, using bacteriostatic water as a diluent for a single-dose medication in a nursing mother is unlikely to pose significant risk, but caution is still advised. If repeated injections (containing bacteriostatic water) are required in a breastfeeding mother, healthcare providers will consider the potential accumulation of benzyl alcohol.
As with pregnancy, an alternative preservative-free diluent may be preferred for nursing mothers if feasible.
Ultimately, decisions on using bacteriostatic water during pregnancy or lactation should involve a risk-benefit assessment by the physician, and therapy should be monitored closely.[9]
Unopened vials of Bacteriostatic Water for Injection have a long shelf life, usually on the order of 2-3 years from the date of manufacture (check the expiration date on the vial).[12]
They should be stored at controlled room temperature, typically around 20°C to 25°C (68°F to 77°F), and kept in a clean, dry place. It is important to protect the vials from excessive heat and direct sunlight during storage, as extreme conditions could potentially degrade the product or its packaging.
The vial should remain sealed and sterile until it is ready to be used. Do not use the solution if you notice any cloudiness, particulate matter, or discoloration, as these could indicate contamination or compromise of the sterile seal.
Under proper storage conditions, an unopened bacteriostatic water vial will remain stable until its printed expiration date.[12]
- RxList. (2021, October 13). Bacteriostatic Water for Injection - Drug Summary. Retrieved from DOI: 10.1097/01.NURSE.0000441246.36711.66
- Wittmer Rejuvenation Clinic. (2025). What Is Bacteriostatic Water and How Is It Used? Retrieved from https://wittmerrejuvenationclinic.com/what-is-bacteriostatic-water-and how-is-it-used/
- SND Staff. (2023, May 16). Bacteriostatic Water 101: Comprehensive Guide to Its Uses and Storage in Healthcare. SyringesNeedlesDepot. Retrieved from https://syringesneedlesdepot.com/blogs/news/bacteriostatic-water-101-comprehensive-guide-to-its-uses-and-storage-in-healthcare
- Pfizer Limited. (2024). Bacteriostatic Water for Injections - Summary of Product Characteristics. electronic Medicines Compendium. Retrieved from https://www.medicines.org.uk/emc/product/6655
- Hospira, Inc. (2019a). Bacteriostatic Water for Injection, USP [Package Insert]. Revised 08/2019. Retrieved from DailyMed: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=87d6e9dc-fe3b-4593-ac9a-d7493d1959c7
- Pfizer Medical Information. (n.d.). Bacteriostatic Water for Injection - Warnings and Precautions. Retrieved from PfizerMedicalInformation.com
- Westend Medical Supplies. (2023, July 15). Bacteriostatic Water: A Comprehensive Understanding. Retrieved from https://westendmedicalsupply.com/blogs/education/bacteriostatic-water-a-comprehensive-understanding
- Hospira, Inc. (2019b). Bacteriostatic Water for Injection - Prescribing Information (Pregnancy Use). Retrieved from DailyMed: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=87d6e9dc-fe3b-4593-ac9a d7493d1959c7
- Pfizer Limited. (2024). Bacteriostatic Water - Use in Pregnancy and Lactation. electronic Medicines Compendium. Retrieved from https://www.medicines.org.uk/emc/product/6655
- Drugs..com. (2024, December 12). Bacteriostatic Water for Injection - Dosage and Administration. Retrieved from https://www.drugs.com/pro/bacteriostatic-water-for-injection.html
- Hospira, Inc. (2019c). Bacteriostatic Water - IV Administration Warning. [Package Insert]. Retrieved from DailyMed: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=87d6e9dc-fe3b-4593-ac9a-d7493d1959c7
- Wittmer Rejuvenation Clinic. (2025). How Long Does Bacteriostatic Water Last After Opening? Retrieved from https://wittmerrejuvenationclinic.com/how-long-does-bacteriostatic-water-last/
- Public Health Ontario. (2020). Updated Guidance on the Use of Multidose Vials (PIDAC IPC Guidance). Retrieved from [nolink]https://www.publichealthontario.ca/en/Health-Topics/IPC/Clinical-Office-Practice/Multidose-Vials[/nolink]
- Wittmer Rejuvenation Clinic. (2025). What Is Bacteriostatic Water and How Is It Used? (Differences from Sterile Water). Retrieved from https://wittmerrejuvenationclinic.com/what-is-bacteriostatic-water-and-how-is-it-used/
- Centers for Disease Control and Prevention. (1982). Neonatal Deaths Associated with Use of Benzyl Alcohol - United States. MMWR, 31(22), 290-291. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/00001109.htm
- Pfizer Medical Information. (n.d.). Bacteriostatic Water - Intravenous Hemolysis Warning. Retrieved from PfizerMedicalInformation.com
- SND Staff. (2023, May 16). Bacteriostatic Water 101: Uses and Storage (Shelf-life after opening). Retrieved from https://syringesneedlesdepot.com/blogs/news/bacteriostatic-water-101-comprehensive-guide-to-its-uses-and-storage-in-healthcare
- Westend Medical Supplies. (2023, July 15). Bacteriostatic Water: A Comprehensive Understanding (Side Effects). Retrieved from
- https://westendmedicalsupply.com/blogs/education/bacteriostatic-water-a-comprehensive-understanding
What is the difference between Bacteriostatic Water and sterile water for injection?
The key difference is the presence of a preservative. Bacteriostatic Water contains 0.9% benzyl alcohol, which inhibits bacterial growth and allows the water to be used in multi-dose vials for up to 28 days after opening.
Sterile water for injection contains no preservative; it is a single-use diluent meant to be used immediately after opening and not stored for reuse.
In practice, bacteriostatic water is used when multiple injections or withdrawals will be needed (thanks to its preservative), whereas sterile water is used when only one-time immediate use is required.
Using sterile water beyond a single use or sharing it between patients can risk contamination, since it has no agent to suppress bacterial growth.[14]
Why is Bacteriostatic Water not used in newborns?
Bacteriostatic Water is avoided in neonates because the benzyl alcohol preservative can be toxic to infants. Newborn babies (especially those born prematurely) lack the mature liver enzymes necessary to metabolize benzyl alcohol, leading to accumulation of this substance in the body.
Even the relatively small amount of benzyl alcohol in bacteriostatic water can build up in neonates and cause a dangerous condition known as “gasping syndrome,” characterized by metabolic acidosis, gasping respiration, respiratory failure, and potentially fatal outcomes.
Due to this risk, any medication that needs diluting for a newborn should be prepared with preservative-free sterile water. In fact, the product labeling for bacteriostatic water includes a warning: **“Not for use in neonates.” **[15]
Can I inject Bacteriostatic Water by itself (without mixing it with medication)?
No. Bacteriostatic Water for Injection must never be injected on its own without being used to dissolve or dilute a medication. It is not intended to treat any condition by itself; it contains no active drug.
Moreover, injecting plain water into the bloodstream can be dangerous: without solutes, it is not isotonic to human blood and can cause red blood cells to burst (hemolysis).
The correct use of bacteriostatic water is to follow a medication’s instructions for dilution. This usually means adding the water to a drug powder or concentrate so that the final solution is safe for injection.
Always ensure that the mixture you inject is appropriately diluted; administering bacteriostatic water alone or in excessive volume could result in serious complications (such as hemolysis or fluid imbalance).[16]
What are the common side effects of using Bacteriostatic Water? Bacteriostatic water itself causes very few side effects. Because it’s just a diluent, most potential issues relate to the injection process or the drug you’re injecting rather than the water.
You might experience some brief stinging or burning at the injection site, or minor irritation. Using proper technique (cleaning the skin, using a sharp needle, etc.) minimizes these effects. In rare cases, repeated injections at the same site can cause tissue irritation or muscle pain.
Serious side effects are uncommon; however, if bacteriostatic water is contaminated or not used correctly, it could contribute to injection site infections, swelling, or abscess formation.
Systemic allergic reactions to the benzyl alcohol in bacteriostatic water are extremely rare, especially given the small amounts used, but as with any injection, if you were to develop symptoms like hives, difficulty breathing, or low blood pressure after an injection, seek medical care.
Generally, when bacteriostatic water is used as intended, patients tolerate it very well and any discomfort is usually mild and temporary.[18]
Do I need a prescription to buy Bacteriostatic Water?
In the United States, bacteriostatic water for injection is classified as a prescription-only product. It is not available over the counter and must be dispensed by a licensed pharmacy upon a valid prescription from a healthcare provider.
This requirement is in place because bacteriostatic water is used to dilute or reconstitute injectable medications, and improper use without medical supervision can pose significant health risks.
While it is not a controlled substance, and its use is common in clinical and home care settings, federal law mandates that it be labeled and sold as “Rx only.”
Always obtain bacteriostatic water from a licensed and reputable source, and use it strictly as directed under medical guidance.[18]
Administration Instructions
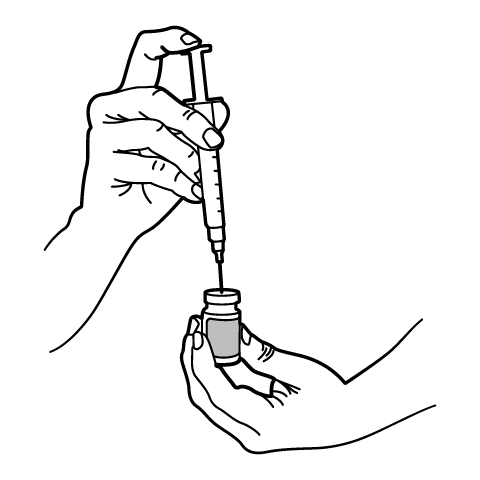
Reconstitution Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Ketorolac Tromethamine Injection
Ketorolac Tromethamine Injection Metoclopramide HCl Injection
Metoclopramide HCl Injection Ondansetron Injection
Ondansetron Injection Sodium Bicarbonate Injection
Sodium Bicarbonate Injection Lidocaine HCl Injection
Lidocaine HCl Injection Ketamine HCL Injection
Ketamine HCL Injection Coenzyme Q10 (Ubidecarenone) Injection
Coenzyme Q10 (Ubidecarenone) Injection Alpha-Lipoic Acid Injection
Alpha-Lipoic Acid Injection Diphenhydramine Injection
Diphenhydramine Injection