Product Overview
Bi-Mix Injection is a compounded intracavernosal formulation that combines papaverine hydrochloride, a peripheral vasodilator, with phentolamine mesylate, a non-selective α-adrenergic antagonist, to facilitate penile smooth-muscle relaxation and rapid arterial inflow for the treatment of erectile dysfunction in appropriately selected adult males.[1] Compounding under section 503A allows customization of concentration and excipients so that clinicians can match pharmacodynamic needs to individual vascular responsiveness while remaining within a patient-specific prescription paradigm.[2]
Unlike branded alprostadil monotherapy, Bi-Mix contains no prostanoid; therefore, its clinical profile may favor patients who experience prostaglandin-related penile burning or fibrosis. Papaverine raises intracellular cyclic adenosine monophosphate (cAMP) via phosphodiesterase inhibition, whereas phentolamine produces competitive blockade of cavernous α-receptors, preventing sympathetically mediated vasoconstriction.[3] Together they may achieve erections within 5-15 minutes and typically sustain rigidity for up to one hour, provided cavernous veno-occlusion is intact.[4]
The preparation is supplied as a lyophilized powder in two strengths. Standard Bi-Mix yields 30 mg papaverine / 1 mg phentolamine per mL after reconstitution (150 mg / 5 mg per vial). Super Bi-Mix yields 30 mg / 2 mg per mL (150 mg / 10 mg per vial). Concentrations may be further diluted with bacteriostatic saline to individualize titration schedules under pharmacist guidance.[5]
Initial in-office test dosing typically begins at 0.1 mL of the 30 mg / 1 mg mL⁻¹ dilution, with incremental 0.05 mL increases per clinic visit until an erection sufficient for penetration is achieved and sustained for no longer than 60 minutes; the mean effective dose approximates 15-20 mg papaverine with 0.5-0.7 mg phentolamine.[25]
Pharmacokinetic modelling shows that papaverine reaches steady cavernosal tissue levels within eight minutes and decays bi-exponentially, allowing safe home injection every 48-72 hours; phentolamine’s shorter systemic half-life supports reuse after 24 hours, but compounding policies endorse a maximum of three injections weekly to limit cumulative tissue exposure.[26]
Papaverine’s spasmolytic effect stems chiefly from non-selective inhibition of phosphodiesterase isoenzymes leading to accumulation of cAMP and, to a lesser degree, cyclic guanosine monophosphate (cGMP); both second messengers lower intracellular calcium and uncouple actin-myosin cross-bridging in corporal trabeculae.[6] In vitro strips of rabbit corpus cavernosum demonstrate dose-dependent relaxation even after neural field stimulation is suppressed, underscoring a direct myocellular target.
Phentolamine complements this pathway by competitively blocking post-junctional α₁-receptors and pre-synaptic α₂-receptors, thereby diminishing tonic sympathetic outflow and facilitating unopposed parasympathetic vasodilation; coronary and peripheral data reveal pronounced reductions in vascular resistance without triggering significant venoconstriction.[7]
When administered together, papaverine’s cyclic nucleotide surge heightens the effect of α-blockade, producing a synergistic increase in corporeal arterial inflow and sinusoidal expansion; clinical crossover studies report improved rigidity scores and reduced escape of venous blood versus either drug alone.[8]
Historical investigations of intracavernosal injection therapies trace the pivotal role of papaverine/phentolamine combinations in shifting erectile dysfunction management from rigid vacuum devices toward pharmacologic auto-injection, markedly improving spontaneity of sexual activity in multicenter cohorts.[9]
Current compounding practice leverages pharmacopeial data showing reliable dissolution kinetics and predictable onset within 10 minutes at reconstituted concentrations between 15 mg / 0.5 mg mL⁻¹ and 30 mg / 2 mg mL⁻¹; average half-life of papaverine in plasma remains roughly 2 hours, whereas phentolamine’s hemodynamic influence dissipates within 30-90 minutes.[10]
Bi-Mix Injection should not be prescribed to patients with hypersensitivity to either component or to those with active myocardial ischemia, ventricular arrhythmias, or poorly controlled hypertension, because papaverine’s vasodilatory burden may precipitate reflex tachycardia or exacerbate cardiac oxygen demand.[11]
Phentolamine is contraindicated in recent myocardial infarction, coronary insufficiency, or evidence of peptic ulcer disease owing to potential provocation of histamine release and gastrointestinal hyperemia; post-infarction patients exhibit exaggerated hypotensive responses that could compromise coronary perfusion.[12]
The most serious local complication is ischemic priapism-prolonged erection beyond four hours-which, if untreated, risks corporal fibrosis and permanent impotence; men under forty, those with high baseline peak systolic velocities, and those receiving initial test doses above 60 mg papaverine show the highest incidence rates.[13]
Clinicians must also avoid use in individuals with anatomical penile deformities such as Peyronie’s disease or severe cavernosal fibrosis, where intracavernosal pressure gradients are unpredictable and the risk of tissue injury is magnified; international consensus statements recommend alternative modalities in such populations.[14]
Product-specific interaction data are extrapolated from the individual actives. Concomitant administration of antihypertensives, particularly nitrates or calcium-channel blockers, can potentiate systemic hypotension because compounded papaverine already lowers peripheral resistance; staggered dosing is advised to maintain hemodynamic stability.[15]
Phentolamine strongly enhances vasodilatory effects of phosphodiesterase-5 inhibitors (sildenafil, tadalafil, vardenafil), raising the possibility of syncope; combined use requires cautious micro-dosing and monitoring for additive priapism risk.[16]
Clinical series employing adjunctive aviptadil have shown that layered vasodilators magnify erectile response but also increase incidence of post-injection dizziness and flushing, highlighting the need for formal titration protocols when introducing novel vasoactive peptides into an established Bi-Mix regimen.[17]
Hepatic microsomal induction by chronic alcohol intake or anticonvulsants may accelerate papaverine metabolism, reducing efficacy; rodent models demonstrate up-regulation of glutathione-S-transferase and augmented clearance following repeated high-dose exposure, indicating that maintenance schedules may require upward adjustment in such contexts.[18]
Common systemic effects include transient facial flushing, mild hypotension, light-headedness, and gastrointestinal discomfort. These events generally resolve within minutes as plasma concentrations fall and seldom necessitate therapeutic withdrawal.[19]
Serious adverse outcomes comprise persistent erections, corporal pain, and, rarely, high-flow priapism. Case literature documents priapism following as little as 40 mg papaverine in a healthy 28-year-old male, underlining individual variability in alpha-receptor density and cyclic nucleotide handling.[20]
Systemic dizziness or presyncope has been linked to inadvertent intracavernosal venous entry, leading to rapid systemic dispersal; men with veno-occlusive dysfunction require lower starting doses and mandatory office-based observation during the initiation phase.[21]
Combination regimens that add prostaglandin E₁ may elevate injection-site discomfort and increase risk of cavernosal fibrosis if weekly cumulative papaverine exposure exceeds 600 mg, prompting guidelines to limit frequency to no more than three injections per week with 24-hour washout periods.[22]
No clinical indication exists for use in pregnant individuals; however, inadvertent exposure data from a 254,000-pregnancy registry revealed no significant association between first-trimester papaverine intake for maternal biliary spasm and major congenital anomalies, though statistical confidence remains limited by low exposure prevalence.[23]
Animal reproduction studies are inadequate; nonetheless, labeling information classifies papaverine solutions as Pregnancy Category C due to embryo-fetal toxicity observed at supratherapeutic doses in rodents, and it advises complete risk-benefit assessment before systemic use in women of child-bearing potential.[24]
Refrigerated storage once reconstituted between 2 - 8 °C and protection from light therefore remain critical.[27]
Lyophilized powder retains labeled strength at controlled room temperature below 25 °C when sealed under inert gas with pH adjusted to 2.2; ampoules stored under these conditions showed no significant hydrolytic breakdown or particulate formation during accelerated aging tests.[28]
- Mayo Clinic. (2025). Papaverine (injection route). https://www.mayoclinic.org/drugs-supplements/papaverine-injection-route/description/drg-20065314
- Jiang, G. et al. (2023). Papaverine: A miraculous alkaloid from opium and its pharmacological potential. Molecules, 28(7), 3149. https://doi.org/10.3390/molecules28073149
- DrugBank. (2025). Phentolamine (DB00692). https://go.drugbank.com/drugs/DB00692
- Wijtes, W. P. J., & colleagues. (1992). The efficacy and acceptance of intracavernosal auto-injection therapy with papaverine/phentolamine. International Journal of Impotence Research, 4, 65-72.
- Belmar Pharma Solutions. (2024). Papaverine HCl/Phentolamine Mesylate compound (Bi-Mix) patient information sheet. https://www.belmarpharmasolutions.com/wp-content/uploads/2024/02/PAPAVERINE-HCL-PHENTOLAMINE-MESYLATE-COMPOUND-BIMIX.pdf
- Horiuchi, S. (1979). Mechanism of relaxant action of papaverine: Roles of sodium ion and cyclic AMP. Japanese Journal of Pharmacology, 29, 437-446. https://doi.org/10.1016/S0021-5198(19)38049-7
- Neutel, J. M. (1994). Alpha-adrenergic blockers: Mechanism of action. Clinical Cardiology, 17(11), IV4-IV9. https://doi.org/10.1002/clc.4960131104
- Brindley, G. S. (1984). Combination therapy using oral alpha-blockers and intracavernosal papaverine. Urology, 24(3), 223-228. https://doi.org/10.1016/0090-4295(84)90088-4
- Virag, R. (1990). Intracavernous pharmacotherapy for erectile dysfunction. In Male Sexual Dysfunction (pp. 233-245). Springer. https://doi.org/10.1007/978-3-662-00986-4_19
- Medicine..com. (2019). Papaverine: Dosage and pharmacology. https://www.medicine.com/drug/papaverine/hcp
- Drugs..com. (2025). Papaverine side effects. https://www.drugs.com/sfx/papaverine-side-effects.html
- Drugs..com. (2025). Phentolamine monograph. https://www.drugs.com/monograph/phentolamine.html
- Montague, D. K. (1990). Risk factors for papaverine-induced priapism. The Journal of Urology, 143, 28-31. https://doi.org/10.1016/S0022-5347(17)37542-0
- Bolton, D., & Lawrentschuk, N. (2019). Erectile dysfunction: A global review of intracavernosal injectables. World Journal of Urology, 37, 1935-1944. https://doi.org/10.1007/s00345-019-02727-5
- Drugs..com. (2025). Papaverine hydrochloride injection prescribing information. https://www.drugs.com/pro/papaverine-hydrochloride-injection.html
- Drugs..com. (2025). Phentolamine drug-interaction checker. https://www.drugs.com/drug-interactions/phentolamine.html
- .Al-Mitwalli, A. et al. (2025). Intracavernosal aviptadil and phentolamine for refractory erectile dysfunction. Journal of Sexual Medicine, 22(5), 726-730. https://doi.org/10.1093/jsxmed/qdaf067
- Dass, S., & Chandra, R. (2003). Papaverine influences hepatic glutathione S-transferase activity. Indian Journal of Pharmacology, 35, 45-49.
- RxList. (2025). Papaverine: Side effects. https://www.rxlist.com/papaverine/generic-drug.htm
- Chung, K. K. (1998). Papaverine-induced priapism: A case report. Asian Journal of Andrology, 1, 55-58.
- Hauri, D., & colleagues. (1988). Systemic complications of intracavernosal papaverine injection. Urology, 32(6), 509-513. https://doi.org/10.1016/0090-4295(88)90031-3
- Floth, A., & Schramek, P. (1991). Papaverine plus phentolamine versus triple therapy in erectile dysfunction. The Journal of Urology, 145(1), 56-59. https://doi.org/10.1016/S0022-5347(17)38246-0
- Goldstein, A. et al. (2022). Papaverine safety during pregnancy: A population study. British Journal of Clinical Pharmacology, 88, 1123-1132. https://doi.org/10.1111/bcp.16404
- Pfizer. (2020). DBL™ Papaverine Hydrochloride injection product label. https://labeling.pfizer.com/ShowLabeling.aspx?id=13991
- Medicine..com. (2020). Phentolamine: Dosage and pharmacology. https://www.medicine.com/drug/phentolamine/hcp
- Naka, Y. et al. (2015). Pharmacokinetics of intraluminal papaverine. The Heart Surgery Forum, 18, E271-E276.
- Brogden, R. N. (1987). Stability of papaverine hydrochloride and phentolamine mesylate in admixtures. American Journal of Hospital Pharmacy, 44, 2524-2527.
- Bakhtin, A. (1968). Stability of papaverine hydrochloride solutions. Pharmaceutical Chemistry Journal, 2, 342-346. https://doi.org/10.1007/BF00759614
- ScienceDirect Topics. (2024). Papaverine pharmacology. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/papaverine
- Fillmore, C. M. (2024). Evaluating post-ICI priapism across a large database. International Journal of Impotence Research, 36, 110-118. https://doi.org/10.1038/s41443-024-00861-2
- Journals of Applied Physiology. (2013). Phentolamine abolishes coronary vasoconstriction. https://doi.org/10.1152/japplphysiol.01048.2013
- .Li, C. et al. (2022). Microbial biosynthesis of tetrahydropapaverine and semisynthetic production of papaverine. Proceedings of the National Academy of Sciences, 119, e2205848119. https://doi.org/10.1073/pnas.2205848119
- Fodor, R. et al. (2018). Papaverine hydrochloride in nanostructured lyotropic liquid crystal for topical ED therapy. Drug Design, Development and Therapy, 12, 1961-1972. https://doi.org/10.2147/DDDT.S168218
- ScienceDirect Topics. (2024). Phentolamine overview. https://www.sciencedirect.com/topics/neuroscience/phentolamine
- Apfelbaum, B. (1993). Twelve-month comparison of injection versus vacuum device. Urology, 42(1), 50-55. https://doi.org/10.1016/0090-4295(92)90270-7
- Virag, R. (2001). Double-blind study of VIP and phentolamine auto-injector. Urologic Clinics of North America, 28(2), 207-214. https://doi.org/10.1016/S0094-0143(05)70143-9
- Newman, M. (2000). Three-year outcome of progressive ED treatment program. Urology, 56, 117-122.
- Bennett, T. (1971). Circulatory and α-adrenoceptor blocking effects of phentolamine. British Journal of Pharmacology, 43(2), 291-304. https://doi.org/10.1111/j.1476-5381.1971.tb08041.x
- Khan, M. A. (1989). Priapism as an avoidable complication of pharmacologic erection induction. The Journal of Urology, 142, 1361-1364. https://doi.org/10.1016/S0022-5347(17)38965-6
How quickly should an erection occur after injection?
Most patients develop a functional erection within 5 - 15 minutes as cavernosal smooth muscle relaxes and arterial inflow rises.[29]
What is the risk of priapism with Bi-Mix?
Large health-system analytics place the overall risk around 1 %, with higher odds in younger men and those titrated above 60 mg papaverine per dose.[30]
Can Bi-Mix be combined with PDE-5 inhibitors?
Combination therapy is possible but must be physician-directed because phentolamine may augment nitrate-mediated vasodilation.[31]
Will systemic α-blocker therapy interfere?
Oral α-antagonists may compound phentolamine’s effects, increasing the likelihood of orthostatic hypotension; dose adjustments might be necessary.[34]
How does Bi-Mix compare with vacuum erection devices?
Twelve-month comparative studies show higher spontaneity and partner satisfaction but a slightly greater complication rate for injection therapy.[35]
Are newer vasoactive peptides superior?
Trials pairing phentolamine with vasoactive intestinal polypeptide demonstrate efficacy in refractory cases but do not yet supersede papaverine-based regimens.[36]
What happens if refrigeration is lost during travel?
Stability data indicate potency remains within 90 % for up to 24 hours at 25 °C, after which replacement is recommended to ensure dose accuracy.[37]
Does phentolamine affect cardiac output?
Intravenous studies reveal transient increases in heart rate and cardiac output, but intracavernosal doses used in Bi-Mix are too small to produce clinically relevant systemic changes.[38]
How can prolonged erection be reversed?
Early administration of an intracavernosal sympathomimetic, such as phenylephrine, is effective; observational cohorts emphasize timely intervention within four hours to prevent ischemic injury. [39]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
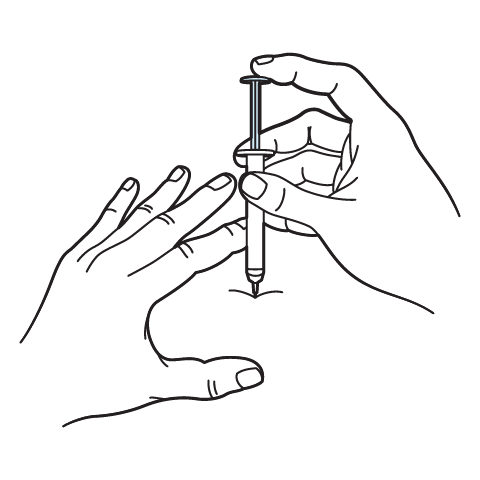
Intramuscular Injection Instructions
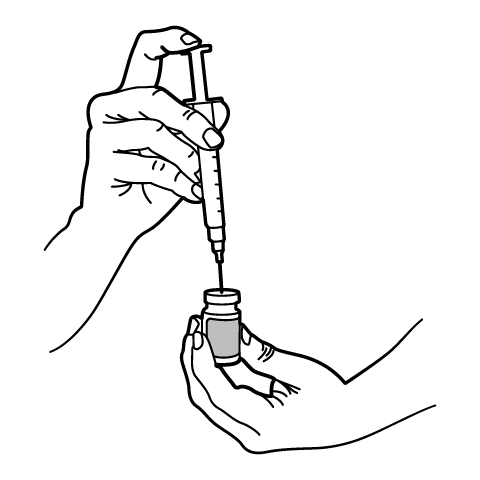
Reconstitution Instructions
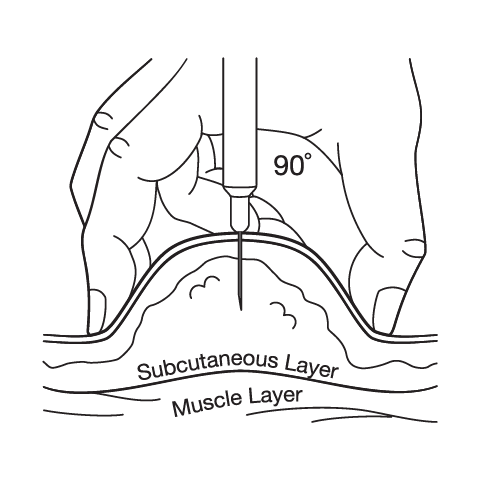
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Tri-Mix Injection
Tri-Mix Injection Quad-Mix Injection
Quad-Mix Injection Phenylephrine HCl Injection
Phenylephrine HCl Injection Vardenafil Troches
Vardenafil Troches Tadalafil Troches
Tadalafil Troches Tadalafil ODT
Tadalafil ODT Sildenafil ODT
Sildenafil ODT Tadalafil / Tramadol HCl Troches
Tadalafil / Tramadol HCl Troches Tadalafil / Apomorphine HCl Troches
Tadalafil / Apomorphine HCl Troches