Product Overview
† commercial product
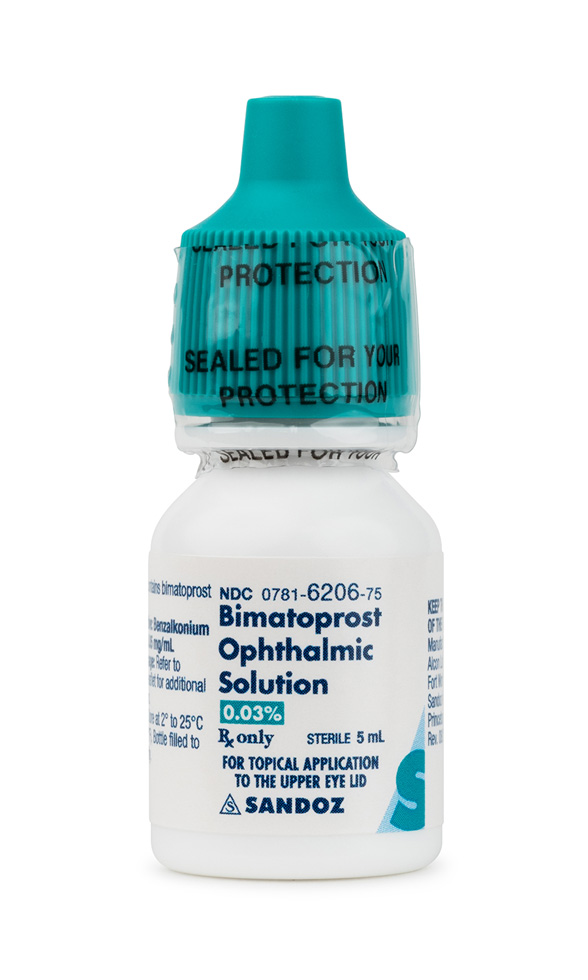
Bimatoprost Eyelash Solution is a sterile, preservative-free topical preparation containing 0.03 % bimatoprost, a synthetic prostaglandin F₂α analogue formulated for nightly dermal application to the upper eyelid margin. Although the active pharmaceutical ingredient (API) has been commercially marketed for eyelash hypotrichosis, the present product is compounded under Section 503A and is therefore dispensed pursuant to an individual, patient-specific prescription; it is not manufactured on a commercial or FDA-approved basis and should be considered an unapproved drug product.
Bimatoprost Eyelash Solution is supplied in 3 mL and 5 mL single-patient containers with disposable applicators and is intended to increase lash length, thickness, and pigmentation by promoting follicles into, and prolonging, the anagen phase of growth. Clinical trials of the reference 0.03% solution demonstrated visible lash enhancement within sixteen weeks, but therapeutic response varies and may regress after discontinuation.
Prescribers should counsel patients regarding potential local adverse effects, including hyperemia, periocular skin darkening, iris pigmentation changes, and periorbital fat atrophy, as well as the need for meticulous application technique to avoid unintended hair growth on adjacent skin.
Because systemic exposure after dermal use is negligible, systemic adverse reactions are uncommon, yet appropriate medical history (e.g., glaucoma therapy, inflammatory eye disease) remains essential.
This compounded formulation must be prescribed and monitored by healthcare professionals familiar with prostaglandin-based therapies and the additional regulatory considerations that accompany 503A compounding.[1]
The recommended regimen is one drop of 0.03% bimatoprost placed on the single-use sterile applicator and stroked gently along the upper eyelid margin once nightly. Excess solution should be blotted, and the applicator discarded after each use to prevent microbial contamination. Application to the lower lid is contraindicated due to the risk of hair growth beyond the lash line. Patients should cleanse the face and remove contact lenses or cosmetics before dosing.
Clinical trial data suggest visible improvements after eight weeks, with maximal cosmetic effect at sixteen weeks; continued nightly application is needed to sustain results, as lash parameters gradually return to baseline over 4-8 weeks after cessation.
Use more than once daily does not enhance efficacy and may increase adverse effects.[7]
Bimatoprost is an amide prodrug that, after percutaneous penetration, binds selectively to the prostamide FP receptor complex expressed in hair follicle dermal papillae and epithelial cells. Activation of these G-protein-coupled receptors increases intracellular free calcium, up-regulates mitogen-activated protein kinase signalling, and ultimately promotes transition from telogen to anagen while lengthening the anagen interval itself.
Histochemical studies have revealed accelerated matrix keratinocyte proliferation and augmented synthesis of keratin-associated proteins, correlating with clinically observable increases in lash length and caliber. Concomitantly, bimatoprost augments melanogenesis within follicular melanocytes, accounting for the darkening effect frequently noted by patients.
Unlike ophthalmic administration, dermal application produces minimal conjunctival hyperemia because vascular FP-receptor density is lower in eyelid skin. Peak plasma levels (< 0.1 ng/mL) occur within ten minutes, and the molecule is rapidly hydrolyzed to an inactive acid metabolite, with elimination half-life under forty minutes; consequently, systemic pharmacodynamic effects, such as reduction of intraocular pressure, are improbable.
Importantly, receptor desensitization has not been demonstrated over twelve-month continuous use, supporting the chronic nightly regimen required for lash maintenance. Nevertheless, precise molecular intermediaries remain incompletely elucidated, and interindividual variability suggests that genetic or epigenetic modulators of follicular FP receptor expression may exist.[2]
Use of bimatoprost Eyelash Solution is contraindicated in any patient with a known hypersensitivity to bimatoprost or formulation excipients. Clinicians should defer initiation in individuals exhibiting active ocular surface inflammation, uveitis, cystoid macular oedema, or iritis, because prostaglandin analogues may exacerbate inflammatory eye disease.
Relative contraindications include aphakic or pseudophakic patients with a torn posterior lens capsule, those with prior complicated ocular surgery, and individuals with a history of herpetic keratitis, as corneal inflammation may be potentiated.
Because dermal application can still result in trace trans-conjunctival migration, caution is advised in glaucoma patients concurrently receiving prostaglandin drops to avoid additive hyperemia or misinterpretation of ocular hypertension control.
Patients using contact lenses should remove them before application and wait at least fifteen minutes before reinsertion to reduce benzalkonium chloride absorption when it is present in concurrent ocular preparations.
No pediatric safety data exist; therefore, therapy in patients under eighteen should be reserved for compelling indications and specialist oversight.
Finally, as permanent iris pigmentation has been documented after ocular exposure, practitioners should weigh the cosmetic benefit against the risk of heterochromia, particularly in individuals with mixed-colored irises.[3]
Dermal dosing yields plasma concentrations several orders of magnitude below those required to influence cytochrome P450 isozymes; clinically meaningful systemic drug-drug interactions are therefore unlikely.
Nevertheless, additive local adverse reactions may occur when prostaglandin analogues are applied concomitantly to the ocular surface, potentially increasing the incidence of conjunctival hyperemia, pruritus, and periocular pigmentation.
Beta-adrenergic antagonists, carbonic anhydrase inhibitors, or alpha agonists used ophthalmically do not appear to modify bimatoprost’s follicular effects, but combination therapy can complicate causality assessment for periocular skin changes.
Systemic absorption of topical β-blockers has been reported to potentiate hypotension and bradycardia; while bimatoprost itself lacks these effects, polypharmacy in glaucoma underscores the need for thorough medication reconciliation.
No interaction studies involving dermal bimatoprost and dermatologic agents, such as retinoids, have been conducted, but concurrent use of skin-irritating preparations could theoretically heighten local erythema.
Because bimatoprost is a weak prostaglandin F₂α analogue, theoretical antagonism with non-steroidal anti-inflammatory drugs exists, yet available pharmacovigilance databases have not confirmed diminished efficacy in users of topical NSAIDs.[4]
The most frequently observed adverse event is mild, transient pruritus or erythema at the application site, occurring in up to 4% of subjects; symptoms generally abate with continued use or improved applicator hygiene.
Periocular skin and lash hyperpigmentation, mediated by increased melanogenesis, develops gradually and may persist even after discontinuation. Hypertrichosis extending to the malar or temporal regions has been reported when solution migrates beyond the lid margin.
Conjunctival hyperemia, punctate keratitis, and dry eye sensation are uncommon but warrant evaluation to exclude inadvertent intraocular exposure.
Iris color change has been documented in less than 1% of dermal users, substantially lower than with ophthalmic dosing, but the alteration is typically permanent.
Rare systemic reactions-including headache, upper respiratory tract symptoms, and dizziness-have been reported but lack definitive causality. There are isolated case reports of periocular fat atrophy leading to deepening of the upper sulcus; pathogenesis may involve prostaglandin-mediated inhibition of adipogenesis.
Patients should be counselled to discontinue use and seek medical review if severe ocular pain, vision changes, or marked inflammation develops.[5]
Bimatoprost is classified as Pregnancy Category C because animal reproduction studies have shown dose-dependent fetal loss and skeletal variations at exposures significantly exceeding human ocular doses, while adequate, well-controlled studies in pregnant persons are lacking.
Dermal application of 0.03 % solution delivers systemic exposures markedly below those associated with adverse developmental findings in rats and rabbits; however, the margin of safety remains indeterminate for the first trimester, when prostaglandin analogues could theoretically influence uterine tone.
Therefore, therapy should be considered only when the potential aesthetic benefit justifies the possible risk to the fetus, and patients should be advised to discontinue treatment upon confirmed pregnancy.
No data exist regarding bimatoprost presence in human milk following dermal application, but animal models demonstrate excretion into milk after parenteral dosing; lactating individuals should weigh the cosmetic benefit against unknown infant exposure.[6]
Bimatoprost Eyelash Solution is chemically stable at controlled room temperature (20 - 25 °C; and should be safeguarded from excessive heat or direct sunlight.
Refrigeration is unnecessary and may lead to increased viscosity that impairs uniform dispensing. The container should remain tightly closed to minimize oxidative degradation and microbial ingress.
Under recommended conditions, unopened vials retain potency until the labelled beyond-use date assigned by the compounding pharmacy, typically six months from preparation.
Opened containers should be discarded after twelve weeks to mitigate contamination risk.[8]
- U.S. Food and Drug Administration. (2021). Latisse (bimatoprost) [Package insert]. [nolink]https://accessdata.fda.gov/drugsatfda_docs/label/2021/022369s014lbl.pdf[/nolink]
- Smith, S., Fagien, S., Whitcup, S. M., et al. (2012). Eyelash growth in subjects treated with bimatoprost: A multicenter, randomized study. Journal of the American Academy of Dermatology, 66(1). https://doi.org/10.1016/j.jaad.2011.10.019
- Review of Optometry. (2016, March 4). Practice Pearl of the Week: Glaucoma meds- side effects and contraindications.
- https://www.reviewofoptometry.com/email/pearl198.html
- UK Clinical Pharmacy Association. (2023). Bimatoprost-Perioperative medicines handbook. https://periop-handbook.ukclinicalpharmacy.org/drug/bimatoprost/
- Drugs..com. (2025, January 11). Bimatoprost ophthalmic side effects. https://www.drugs.com/sfx/bimatoprost-ophthalmic-side-effects.html
- Drugs..com. (2025). Bimatoprost ophthalmic use during pregnancy. https://www.drugs.com/pregnancy/bimatoprost-ophthalmic.html
- DailyMed. (2024). Bimatoprost ophthalmic solution: Dosage and administration. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=27bef7e1-750a-4ac1-ab5f-e4c0121ffcbc
- Chatterjee, A., & Sarkar, S. (2008). Comparative stability of bimatoprost 0.03 % and latanoprost 0.005 %. BMC Ophthalmology, 8, 11. https://doi.org/10.1186/1471-2415-8-11
- Goldberg, D. F., et al. (2013). Patient-reported outcomes after bimatoprost for eyelash hypotrichosis. Aesthetic Surgery Journal, 33(6), 789-798. https://doi.org/10.1093/asj/sjt050
- Mahadevan, R., & Singh, P. (2014). Bimatoprost-induced iris hyperpigmentation: Case report. Clinical Therapeutics, 36(2), e7-e9. https://cdn.mdedge.com/files/s3fs-public/CT104002007_e.PDF
- Day, D. G., Jones, S. A., & Whitcup, S. M. (2003). Systemic pharmacokinetics of bimatoprost 0.03 % solution. Investigative Ophthalmology & Visual Science, 44(5), 2173. https://iovs.arvojournals.org/article.aspx?articleid=2418003
- Allergan. (2011). Lumigan (bimatoprost) ophthalmic solution 0.03 % [Package insert]. [nolink]https://accessdata.fda.gov/drugsatfda_docs/label/2011/021275s022lbl.pdf[/nolink]
- Drugs..com. (2025). Bimatoprost monograph for professionals.
- https://www.drugs.com/monograph/bimatoprost.html
- ChemIgnition. (2025). Latanoprost vs bimatoprost: Key differences for formulators. https://chemignition.com/blog/latanoprost-vs-bimatoprost-key-differences
- Singh, K. (2023). Glaucoma myth busters: Prostaglandin analogues and inflammation. Glaucoma Physician. https://glaucomaphysician.net/issues/2023/march/glaucoma-myth-busters-myths-about-prostaglandin-analogues-and-inflammation/
- Harris, M. (2009, January 5). Glaucoma meds repurposed to grow longer lashes. Wired. https://www.wired.com/2009/01/glaucoma-meds-h
- CVS Health. (2014). Latisse prescribing information.
- [nolink]https://www.cvs.com/bizcontent/minuteclinic/wso_slotcontent/services/wellness physicals/latisse-prescribing-info.pdf[/nolink]
- AppliancesFirst. (2025). The truth about bimatoprost: Do you need to refrigerate it? https://appliancesfirst.com/does-bimatoprost-need-to-be-refrigerated/
How soon will patients notice longer lashes?
Clinical data indicate measurable increases in length and fullness by week eight, with optimal cosmetic effect at sixteen weeks of nightly use.[9]
Can iris or eyelid color change really happen?
Yes, prolonged exposure may trigger melanocyte activation, leading to permanent iris darkening or periocular skin hyperpigmentation, though incidence is low with dermal application.[10]
Does the medication enter the bloodstream?
Systemic absorption is minimal; peak plasma levels after dermal use are below 0.1 ng/mL and decline to undetectable within ninety minutes.[11]
Is efficacy reduced if the patient is already using a prostaglandin eye drop for glaucoma?
No formal antagonism exists, but concurrent ophthalmic prostaglandins may increase local side-effect burden; counselling on application technique mitigates overlap.[12]
What happens if a dose is missed?
Skip the missed application and resume the regular nightly schedule; doubling the next dose offers no added benefit and may heighten irritation.[13]
Are there formulation differences between bimatoprost and latanoprost serums sold online?
Bimatoprost is a prostamide with greater receptor affinity and proven lash-growth data, whereas latanoprost is an isopropyl ester with limited cosmetic
evidence.[14]
Does bimatoprost worsen ocular inflammation?
Large cohort analyses suggest prostaglandin analogues rarely precipitate uveitis; nevertheless, patients with active intraocular inflammation should defer therapy.[15]
Why was bimatoprost originally a glaucoma drug?
Investigators noted lash growth as an incidental finding during glaucoma trials, leading to repurposing for hypotrichosis.[16]
Do patients need an in-person prescription each refill?
Yes; under 503A regulations, each compounded fill requires a patient-specific prescription from an authorized prescriber.[17]
Should the bottle be refrigerated after opening?
No; room-temperature storage preserves chemical stability, whereas refrigeration may promote precipitation and dosing inconsistency.[18]
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 GHK-Cu Scalp Solution
GHK-Cu Scalp Solution GHK-Cu Facial Serum
GHK-Cu Facial Serum Hair Restore Lite Scalp Solution
Hair Restore Lite Scalp Solution Hair Restore Ultra Scalp Solution
Hair Restore Ultra Scalp Solution NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Sermorelin Acetate Injection
Sermorelin Acetate Injection NAD+ Cream
NAD+ Cream Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection Minoxidil Capsules
Minoxidil Capsules