Product Overview
Carnitine (L) Injection is a sterile compounded solution containing Levocarnitine-the biologically active L-isomer of carnitine-at a concentration of 500 mg/mL, dispensed in a 30 mL vial.
Levocarnitine is an amino-acid derivative essential for mitochondrial fatty-acid transport, and this injectable formulation is used to treat or prevent clinically significant carnitine deficiency in conditions such as primary systemic carnitine deficiency, certain inborn errors of metabolism, and end-stage renal disease on dialysis.
By restoring normal carnitine levels, therapy aims to improve energy production, muscle function, and cardiac performance in patients unable to maintain adequate stores through diet or oral supplements.[1]
Dosing is individualized to body weight, severity of deficiency, and clinical context.
In hereditary or acquired metabolic defects, an initial intravenous bolus of 50 mg/kg may be administered over two to three minutes, followed by maintenance doses of 50 mg/kg every six hours or continuous infusion to achieve a total daily exposure of up to 300 mg/kg in critical illness.
Hemodialysis patients commonly receive 10-20 mg/kg intravenously at the end of each dialysis session, with trough free carnitine concentrations guiding subsequent adjustments.
Compounded vials containing 500 mg/mL should be diluted to 2-8 mg/mL in isotonic saline for infusion; intramuscular use is limited to small, divided volumes.
Therapy duration varies from acute correction in metabolic crises to lifelong supplementation in primary systemic deficiency.
Missed doses should not be doubled; instead, resume the prescribed schedule and consult the clinician for guidance.[7]
Levocarnitine exerts its therapeutic effect by serving as a carrier molecule that transports activated long-chain fatty acids across the inner mitochondrial membrane. It forms acyl-carnitine esters with acyl-CoA derivatives, enabling entry into the mitochondrial matrix where β-oxidation generates acetyl-CoA and ultimately ATP.
This shuttle ensures efficient oxidation of fatty acids in energy-dependent tissues such as skeletal muscle and myocardium. In addition, levocarnitine buffers excess intramitochondrial acyl-CoA, regenerating free coenzyme A and potentially preventing inhibition of critical metabolic enzymes.
By facilitating removal of potentially toxic acyl groups and stabilizing cellular membranes, levocarnitine may support metabolic flexibility and protects against accumulation of organic acids.
Replenishing systemic stores may restore normal oxidative capacity and improves metabolic homeostasis in deficient states.[2]
Levocarnitine injection carries no absolute contraindications in FDA labeling; however, administration is inadvisable in any patient with a known hypersensitivity to levocarnitine or formulation components.
Caution is recommended in individuals with seizure disorders, as increased frequency or severity of seizures has been reported during therapy.
Accumulation may occur in patients with severe renal impairment who are not receiving dialysis, necessitating careful monitoring.
Only the L-isomer should be used clinically, because exposure to the racemic D, L-carnitine has produced reversible myasthenia in uremic patients.[3]
Clinically important interactions with levocarnitine are uncommon, but two deserve attention.
Concomitant use with coumarin anticoagulants such as warfarin has been associated with elevations in the international normalized ratio, requiring closer INR surveillance and potential dose adjustment.
High-dose levocarnitine can also antagonize the intracellular actions of thyroxine and triiodothyronine by limiting their nuclear uptake; patients receiving thyroid-hormone replacement or those with thyroid dysfunction should be monitored for changes in symptom control.
Other routinely prescribed drugs, vitamins, and enteral or parenteral nutrition products have not demonstrated significant pharmacokinetic or pharmacodynamic interference.[4]
The most frequently reported adverse effects include transient gastrointestinal discomfort-nausea, vomiting, abdominal cramps, or diarrhea-particularly when large doses are delivered rapidly.
A distinctive fish-like odor of breath, sweat, or urine may emerge as trimethylamine metabolites are excreted; although benign, it can be socially bothersome.
Local pain, erythema, or phlebitis may develop at intravenous or intramuscular injection sites, mitigated by slow administration and site rotation.
Rare but serious events encompass provocation or exacerbation of seizures in susceptible individuals and hypersensitivity reactions ranging from urticaria to anaphylaxis.
Overall, events are usually mild and reversible upon dose adjustment or discontinuation.[5]
Animal reproduction studies have not demonstrated fetal harm with levocarnitine, and the drug is classified as Pregnancy Category B.
Because adequate, well-controlled studies in pregnant individuals are lacking, use should be limited to circumstances in which the potential maternal benefit clearly outweighs theoretical fetal risk-such as treatment of documented systemic carnitine deficiency.
Levocarnitine is a normal constituent of human milk, and supplemental doses may modestly increase breast-milk concentrations; no adverse outcomes have been attributed to nursing infants, yet unnecessary exposure should be avoided.
Prescribers should base decisions on clinical necessity and monitor both mother and infant when use during lactation is unavoidable.[6]
Carnitine (L) Injection should be stored at controlled room temperature-20 to 25 °C (68 to 77 °F)-and protected from light in its original carton.
Do not freeze or expose to excessive heat.
Visual inspection for particulate matter or discoloration is required before administration; compromised vials should be discarded according to local disposal regulations.[8]
- U.S. National Library of Medicine. (2019). Levocarnitine injection, USP [Drug label]. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ea45f7e8-8b7e-460f-9fba-9e1cd5f3a42e
- Flanagan, J. L., Simmons, P. A., Vehige, J., Willcox, M. D. P., & Garrett, Q. (2010). Role of carnitine in fatty acid metabolism and deficiency. Nutrition & Metabolism, 7(1), 30. https://doi.org/10.1186/1743-7075-7-30
- Sigma-Tau Pharmaceuticals. (2018). Carnitor (Levocarnitine) Injection [Package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020157s016lbl.pdf
- Toth, P. P., & Gatlin, L. (2015). Drug interactions between levocarnitine and anticoagulants: A review of pharmacodynamic considerations. Clinical Therapeutics, 37(12), 2868-2874. https://doi.org/10.1016/j.clinthera.2015.10.028
- U.S. National Library of Medicine. (2019). Levocarnitine injection-Adverse reactions. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ea45f7e8-8b7e-460f-9fba-9e1cd5f3a42e
- Drugs..com. (2024, April 23). Levocarnitine-Pregnancy and breastfeeding warnings. https://www.drugs.com/pregnancy/levocarnitine.html
- U.S. National Library of Medicine. (2019). Levocarnitine injection-Dosage and administration. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ea45f7e8-8b7e-460f-9fba-9e1cd5f3a42e
- U.S. National Library of Medicine. (2019). Levocarnitine injection-Storage and handling. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ea45f7e8-8b7e-460f-9fba-9e1cd5f3a42e
- U.S. National Library of Medicine. (2019). Levocarnitine injection, USP [Drug label]. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cf801cc4-775e-433d-9d32-e5d9a98981d3
- National Institutes of Health, Office of Dietary Supplements. (2023, April 17). Carnitine: Fact sheet for health professionals. https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/
- Sigma-Tau Pharmaceuticals. (2021). Carnitor (Levocarnitine) Injection [Package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020157s018lbl.pdf
- Mayo Clinic. (2024, April 2). Levocarnitine (intravenous route) - Description and brand names. https://www.mayoclinic.org/drugs-supplements/levocarnitine-intravenous-route/description/drg-20064527
- Cleveland Clinic. (2024). Levocarnitine injection: Patient education. https://my.clevelandclinic.org/health/drugs/20823-levocarnitine-injection
- RxList. (2024). Levocarnitine (Carnitor) - Side effects and patient information. https://www.rxlist.com/carnitine-drug.htm
- Toth, P. P., & Gatlin, L. (2015). Drug interactions between levocarnitine and anticoagulants: A review of pharmacodynamic considerations. Clinical Therapeutics, 37(12), 2868-2874. https://doi.org/10.1016/j.clinthera.2015.10.028
- Pooyandjoo, M., Nouhi, M., Shab-Bidar, S., Djafarian, K., & Olyaeemanesh, A. (2016). The effect of L-carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews, 17(10), 970-976. https://doi.org/10.1111/obr.12436
- Harvard Health Publishing. (2023, November 15). Supplements and sports performance: What works? https://www.health.harvard.edu/blog/supplements-and-sports-performance-what-works-2023111530
- Vreeburg, R. A., Oudshoorn, A., & Ketting, D. (1995). Carnitine deficiency during pivampicillin therapy in children. Clinical Pharmacology & Therapeutics, 58(6), 685-689. https://doi.org/10.1016/0009-9236(95)90013-6
What exactly is Carnitine (L) Injection and why might I need it?
Carnitine (L) Injection delivers levocarnitine, a nutrient that shuttles long-chain fatty acids into mitochondria so cells can convert fat into energy.[10]
When a clinically significant carnitine deficiency is documented-such as in primary systemic carnitine deficiency, certain inborn errors of metabolism, or patients on chronic hemodialysis-injectable therapy is prescribed because it may restore blood and tissue levels more rapidly than oral supplements.[9]
Who typically receives the injection instead of over-the-counter carnitine pills?
The injectable form is reserved for people whose intestines or kidneys cannot absorb, synthesize, or conserve enough carnitine.[9]
Examples include infants with genetic transporter defects, adults experiencing metabolic crises, and dialysis patients who lose carnitine during each treatment.[10]
In these situations an oral supplement may be inadequate or too slow, so clinicians rely on intravenous or intramuscular levocarnitine.[11]
How is Carnitine (L) Injection administered and how often will I need it?
In medical settings it is usually diluted in normal saline and infused slowly into a vein, with a typical loading dose of 50 mg per kilogram over two to three minutes followed by maintenance doses every six hours or by continuous infusion; patients on hemodialysis often receive 10-20 mg/kg at the end of each session.[11]
Your prescriber will individualize both the route (IV or IM) and the timetable based on your weight, kidney function, and overall clinical picture.[9]
How quickly should I notice an effect?
Plasma free-carnitine levels rise within minutes of an intravenous dose, and many patients report improved energy or reduced muscle fatigue within days to a few weeks once tissue stores are replenished.[11]
What side effects should I watch for?
The most common reactions include transient digestive upset (nausea, vomiting, stomach cramps, or diarrhea), a mild “fishy” body odor, headache, or soreness and redness where the injection is given.[9][13][14]
Rarely, people with a seizure disorder may experience more frequent seizures, and severe allergic reactions have been reported; contact your healthcare provider if you notice anything unusual.[9]
Why can this medication give my breath or sweat a fishy smell?
Levocarnitine can be metabolized to trimethylamine, an amine with a distinctive fish-like odor that is excreted in sweat, breath, and urine; the smell is harmless and often diminishes when dosing is slowed or reduced.[9]
Will Carnitine (L) Injection help me lose weight or boost my gym performance?
Research shows only modest effects. A systematic review of clinical trials found L-carnitine users lost roughly 1-2 kg more than placebo over several months, and evidence for significant performance enhancement is inconsistent.[16][17] People who are already carnitine-deficient may feel less fatigue, but the injection should not be viewed as a stand-alone weight-loss or athletic supplement.
Is it safe to use while I’m pregnant or breastfeeding?
Animal studies have not shown harm to the fetus, and levocarnitine is classified as Pregnancy Category B; injections are used in pregnancy only when the potential benefit clearly outweighs any theoretical risk.[11]
Small, naturally occurring amounts of carnitine pass into breast milk; therapeutic doses have not been linked to problems in nursing infants, but your clinician may monitor both mother and baby if treatment is necessary.[12]
Does it interact with any of my other medicines?
Levocarnitine is generally low-risk, but it has been reported to increase the effect of warfarin, raising the INR, and long-term use of certain pivalate-containing antibiotics can lower body carnitine stores.[15][18]
Always tell your healthcare provider about every medicine and supplement you take so dosing and monitoring can be adjusted.
How should I store my vial, and what if I miss a scheduled dose?
Keep unopened vials at 20-25 °C (68-77 °F) and protect them from light; do not freeze. If you miss a dose, do not double up-resume your regular schedule and consult your prescriber for advice.[9]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
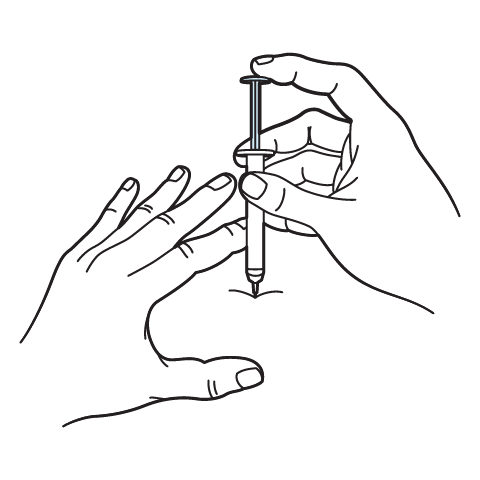
Intramuscular Injection Instructions
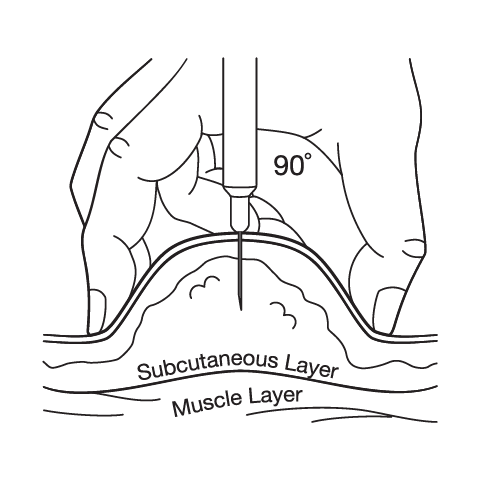
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Lipo-C Injection
Lipo-C Injection Bi-Amino Injection
Bi-Amino Injection BCAA Injection
BCAA Injection Taurine Injection
Taurine Injection Lysine HCL Injection
Lysine HCL Injection Testosterone Cypionate Injection
Testosterone Cypionate Injection Lipo Injection
Lipo Injection Lipo-B Injection
Lipo-B Injection Cholecalciferol (D3) Injection
Cholecalciferol (D3) Injection Coenzyme Q10 (Ubidecarenone) Injection
Coenzyme Q10 (Ubidecarenone) Injection