Product Overview
† commercial product
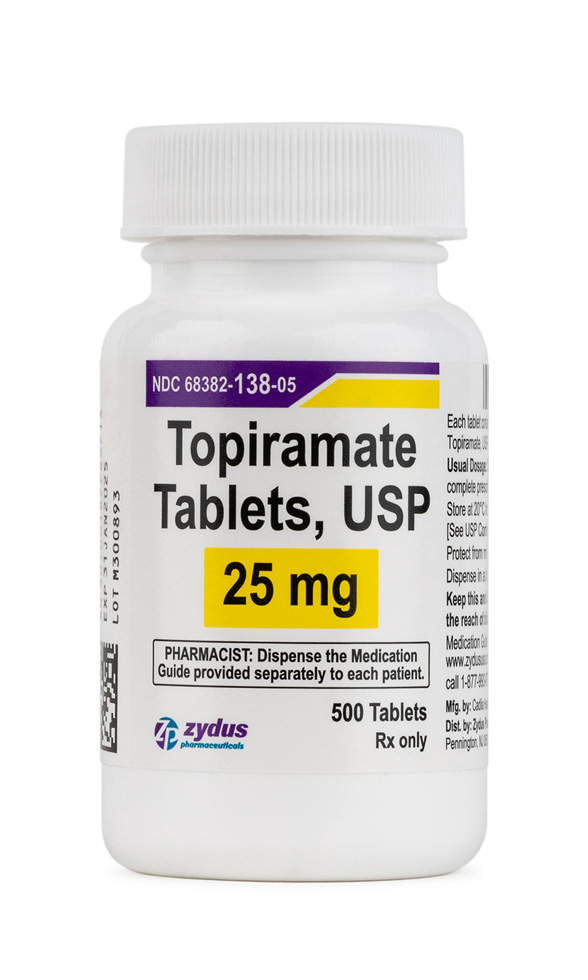
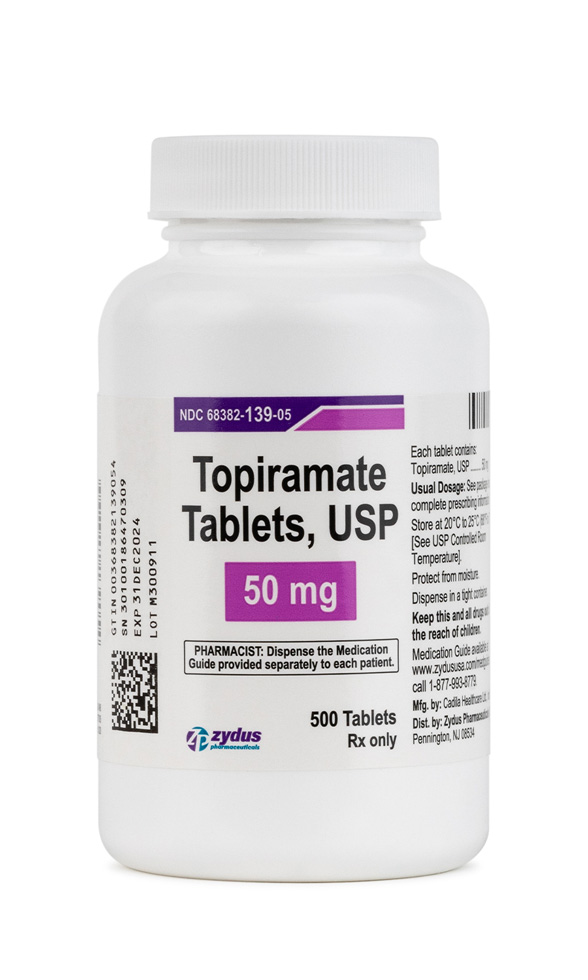
Topiramate is a synthetic, sulfamate-substituted monosaccharide first identified during structure-activity screening for fructose-1,6-bisphosphatase inhibitors and later developed as a broad-spectrum antiseizure medication.[1] Because its multiple ion-channel and neurotransmitter actions dampen pathologic cortical excitability, topiramate received United States marketing clearance in 1996 for monotherapy or adjunctive control of partial-onset and primary generalized tonic-clonic seizures. Subsequent randomized trials demonstrated a clinically meaningful reduction in monthly migraine days, resulting in a separate indication for preventive treatment of migraine in adolescents and adults, while post-approval experience revealed further off-label utility-for example in essential tremor, binge-eating disorder, and antipsychotic-associated weight gain-though such uses remain investigational.[1] The tablet strengths commercially supplied in the United States (25 mg and 50 mg for this compounded analogue) are frequently preferred when slow titration is needed to mitigate paranesthesia, cognitive fog, and dysgeusia, all of which occur in a dose-dependent fashion.[1]
All dispensing of topiramate tablets in the United States requires a prescription from a licensed prescriber; compounded batches prepared under section 503A must follow patient-specific orders and cannot be resold.[2] Because the drug alters serum bicarbonate through weak carbonic-anhydrase inhibition, clinicians generally obtain baseline electrolytes, renal function, and, where clinically indicated, intra-ocular pressure, repeating these tests periodically during long-term therapy. Any compounded preparation should match the labeled strength of the reference product, undergo content-uniformity testing, and be dispensed with both the FDA Medication Guide and the pharmacy’s beyond-use dating statement.[2]
Although topiramate’s pharmacodynamic profile overlaps with older carbonic-anhydrase inhibitors such as acetazolamide, it differs by exhibiting negligible effects on systemic pH at doses below 100 mg per day; above this threshold, however, mild, non-anion-gap metabolic acidosis may emerge, especially in patients with renal impairment or concomitant ketogenic diets.[1][2] The resulting decrease in serum bicarbonate can manifest clinically as fatigue, hyperventilation, or cardiac arrhythmia, underscoring the importance of individualized titration, electrolyte monitoring, and patient education regarding adequate hydration and early reporting of new-onset malaise.[2]
For seizure monotherapy in adults, titration typically begins at 25 mg nightly for one week, increasing by 25-50 mg increments weekly to a target of 100 mg twice daily; doses up to 400 mg daily are occasionally required. Migraine prophylaxis usually responds to 50 mg twice daily, with meaningful benefit often evident at 25 mg twice daily after four weeks.[2] Renal impairment (creatinine clearance <70 mL/min) necessitates halving both titration increments and maintenance doses, while hemodialysis patients require supplemental post-dialysis dosing because 40-50 % of circulating drug may be removed during a four-hour session.[2] When discontinuation is indicated, tapering by 50 mg every week minimizes rebound seizures or migraine exacerbation.[2]
Topiramate’s antiseizure efficacy arises from convergent actions on fast-inactivating voltage-gated sodium channels, high-voltage-activated calcium channels, AMPA/kainate glutamate receptors, and extra synaptic γ-aminobutyric-acid type-A (GABA_A) receptors, each contributing partial but complementary reductions in neuronal firing thresholds.[3] The drug enhances GABA-evoked chloride currents at a non-benzodiazepine modulatory site, stabilizing inhibitory networks without producing classic sedative-hypnotic effects. Simultaneously, antagonism of AMPA/kainate receptors curtails excitotoxic glutamatergic signaling that underlies seizure propagation and cortical spreading depolarizations.[3]
Beyond channel modulation, topiramate allosterically inhibits certain carbonic-anhydrase isoenzymes expressed in renal tubules and ocular ciliary epithelium; this inhibition increases urinary bicarbonate loss, slightly elevates urine pH, and explains both a predisposition to calcium-phosphate nephrolithiasis and the rare idiosyncratic syndrome of acute myopia with secondary angle-closure glaucoma. In migraine prophylaxis, preclinical models suggest that topiramate dampens cortical spreading depression, attenuates trigeminovascular calcitonin-gene-related-peptide release, and down-regulates central nociceptive transmitter traffic-mechanisms distinct from its antiepileptic activity but still rooted in excitatory-inhibitory balance. In the setting of weight management, appetite suppression may relate to hypothalamic AMPA antagonism, while improved insulin sensitivity appears secondary to mitochondrial carbonic-anhydrase blockade and enhanced adiponectin secretion.[4] Collectively, these diverse molecular effects account for the medication’s broad clinical range, dose-responsive tolerability profile, and numerous pharmacodynamic interactions with other central-acting drugs.[3][4]
Absolute contraindications include prior idiosyncratic hypersensitivity to topiramate or any tablet excipient. Because weak carbonic-anhydrase inhibition can precipitate non-anion-gap metabolic acidosis, caution or avoidance is warranted in patients with chronic metabolic acidosis, severe pulmonary disease with chronic hypercapnia, or conditions pre-disposing to osteoporosis, nephrolithiasis, or nephrocalcinosis. Topiramate should not be initiated during an acute narrow-angle glaucoma attack, and its continuation is inadvisable in patients who develop persistent ocular pain or visual blurring suggestive of ciliochoroidal effusion-induced lens displacement. Concomitant administration with ketogenic diets may accentuate acidosis and should be undertaken only with rigorous biochemical surveillance. Finally, topiramate is contraindicated in patients undergoing concomitant metformin therapy in advanced renal failure, as both agents rely on renal clearance and share the risk of lactic acidosis.[5]
Pharmacokinetic interactions are relatively limited because topiramate is neither a strong cytochrome-P450 inducer nor inhibitor at customary doses; however, doses ≥200 mg per day have been shown to lower systemic exposure to ethinyl estradiol by up to 30 %, reducing contraceptive efficacy. Patients using combined hormonal contraceptives therefore require counseling on additional barrier protection, dose escalation of estrogen, or alternative regimens when topiramate exceeds migraine-prophylactic ranges. Renal elimination via tubular secretion can be competitively inhibited by probenecid, raising steady-state topiramate concentrations; conversely, strong P-glycoprotein inducers such as rifampin may decrease levels.[6]
Pharmacodynamic synergy with other carbonic-anhydrase inhibitors (acetazolamide, zonisamide) amplifies the risk of metabolic acidosis and nephrolithiasis, while combined use with valproate has been repeatedly implicated in hyperammonemic encephalopathy even at therapeutic serum valproate levels. Clinicians should monitor serum ammonia if unexplained cognitive decline, lethargy, or vomiting occurs during dual therapy and promptly discontinue one agent.[7] Distinctly, topiramate may potentiate central nervous system depression when co-administered with alcohol, opioids, or benzodiazepines, necessitating cautious dose titration and patient education on operating machinery.[6]
The most frequently reported adverse reactions-paranesthesia of the distal extremities, dysgeusia, somnolence, concentration difficulties, weight loss, and dizziness-occur in up to 40 % of patients during rapid titration but decline substantially with slower dose escalation and hydration. Cognitive slowing manifests as word-finding difficulty or impaired attention and is typically dose-dependent, reversible upon dose reduction, and accentuated by co-administration of other sedatives.[8]
Serious but uncommon events include acute myopia with secondary angle-closure glaucoma, typically emerging within the first two weeks of therapy; prompt drug discontinuation and ophthalmologic management usually reverse the process.[9] Nephrolithiasis develops in approximately 1-2 % of adults, with risk correlated to pre-existing renal tubular acidosis, high-protein diets, and inadequate fluid intake.[8][9] Oligohidrosis and hyperthermia have been observed in pediatric patients during hot weather; caregivers should be instructed to monitor for decreased sweating and maintain fluid intake.[8] Topiramate’s teratogenic risk profile-most notably oral clefts-is addressed in Section 6.
Human pregnancy registries and population-based case-control studies demonstrate a dose-related elevation in the prevalence of cleft lip with or without cleft palate among infants exposed to topiramate monotherapy in the first trimester. The absolute risk remains low (≈1 %) but is several-fold higher than background; counseling should therefore include discussion of alternative therapies, folic-acid supplementation, and detailed mid-trimester anatomy ultrasonography.
Maternal metabolic acidosis may further complicate gestation by impairing fetal oxygen delivery; bicarbonate and electrolyte monitoring is advised throughout pregnancy. Breast-milk concentrations are approximately 50 % of maternal plasma; although no definitive neurodevelopmental toxicity has been proven, nursing mothers should weigh potential risks against seizure control, using the lowest effective dose.[10]
Compounded topiramate tablets should be packaged in tight, light-resistant containers and stored between 20 °C and 25 °C with a permissible excursion of 15 °C-30 °C.[11] Moisture must be controlled to prevent hydrolytic degradation of the sulfamate moiety, and desiccant packets are recommended for multi-dose vials.
- Pearl, N. Z., Babin, C. P., Catalano, N. T., et al. (2023). Narrative review of topiramate: Clinical uses and pharmacological considerations. Advances in Therapy, 40, 3626-3638. https://doi.org/10.1007/s12325-023-02586-y
- A-S Medication Solutions. (2025, June 9). Topiramate tablets, USP [package insert]. DailyMed. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f16b14a2-297f-4adb-8d7a-5f43785faef7
- Bai, Y.-F., Zeng, C., Jia, M., & Xiao, B. (2022). Molecular mechanisms of topiramate and its clinical value in epilepsy. Seizure, 98, 51-56. https://doi.org/10.1016/j.seizure.2022.03.024
- Rafaelli, B., García-Azorín, D., Boucherie, D. M., et al. (2023). European Headache Federation critical reappraisal and meta-analysis of oral drugs in migraine prevention-part 3: topiramate. The Journal of Headache and Pain, 24, 134. https://doi.org/10.1186/s10194-023-01671-5
- Dell’Orto, O., Battisti, A., & Mazzuca, S. (2016). The frequency and severity of metabolic acidosis related to topiramate. Journal of International Medical Research, 44, 1376-1384. https://doi.org/10.1177/0300060516669897
- Rosenfeld, W. E., Doose, D. R., Walker, S. A., & Nayak, R. K. (1997). Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia, 38, 317-323. https://doi.org/10.1111/j.1528-1157.1997.tb01123.x
- Hamer, H. M., Knake, S., Schomburg, U., & Rosenow, F. (2000). Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology, 54, 230-232. https://doi.org/10.1212/WNL.54.1.230
- Doose, D. R., & Walker, S. A. (2008). Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. Epilepsia, 49, 3-9. [nolink]https://doi.org/10.1111/j.1528-3458.2008.00041.x[/nolink]
- Fraunfelder, F. W., & Fraunfelder, F. T. (2004). Acute myopia and angle-closure glaucoma associated with topiramate use. American Journal of Ophthalmology, 137, 193-195. https://doi.org/10.1016/S0002-9394(03)00774-8
- Margulis, A. V., Mitchell, A. A., Gilboa, S. M., et al. (2012). Use of topiramate in pregnancy and risk of oral clefts. American Journal of Obstetrics and Gynecology, 207, 405.e1-405.e7. https://doi.org/10.1016/j.ajog.2012.07.008
- United States Pharmacopeia. (2023). 〈795〉 Pharmaceutical compounding-nonsterile preparations. USP-NF. https://www.usp.org/compounding/general-chapter-795
- Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Can topiramate tablets be split for dose adjustment?
Scored commercial tablets may be halved, but compounded tablets should only be split if content-uniformity testing demonstrates acceptable variability.[1][2]
How soon after starting therapy will migraine prevention be noticeable?
Many patients observe fewer attacks within four weeks once 50 mg daily is reached, though maximal benefit may require three months.[1]
Does topiramate impair fertility?
No direct gonadal toxicity has been demonstrated; any subfertility is usually attributable to underlying neurologic disease or concomitant medications.[1]
Will carbonated beverages taste flat?
Dysgeusia often alters perception of carbonated drinks because carbonic-anhydrase inhibition changes oral pH; flavor usually normalizes after dose reduction or several weeks.[8]
Is weight loss inevitable?
Average weight loss is 2-3 kg over six months, but dietary choices and baseline body-mass index modify individual responses.[4]
Must serum bicarbonate be checked routinely?
At baseline and periodically-especially at doses ≥200 mg daily or in patients with pulmonary disease-to detect subclinical acidosis.[5]
Can I drive while taking topiramate?
Until individual effects on alertness are known, caution is advised; most patients adjust within two weeks if titration is gradual.[8]
Do over-the-counter antacids interfere with topiramate?
Aluminum-magnesium preparations do not meaningfully alter pharmacokinetics, but sodium bicarbonate-containing products may exacerbate metabolic alkalosis and should be used sparingly.[6]
How should missed doses be handled?
Take the forgotten tablet as soon as remembered unless it is within six hours of the next scheduled dose; doubling the next dose is discouraged to avoid paresthesia and confusion.[2]
Is laboratory ammonia monitoring needed if I am not on valproate?
Routine ammonia testing is unnecessary in monotherapy, but any sudden confusion warrants evaluation, particularly when topiramate is combined with valproate.[7]
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Phentermine HCl / Topiramate Capsules
Phentermine HCl / Topiramate Capsules Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection Tirzepatide / Niacinamide Injection
Tirzepatide / Niacinamide Injection Phentermine HCl Capsules
Phentermine HCl Capsules Bella Capsules
Bella Capsules Lipo Sculpt Cream
Lipo Sculpt Cream Lipo-C Injection
Lipo-C Injection Lipo-B Injection
Lipo-B Injection Metformin Synergy Capsules
Metformin Synergy Capsules